UNIT 2
Q.1 What are miller indices?
Answer:
It is necessary to have some method of identifying and visualising the most useful planes. Crystallographers use an identification system referred to as Miller, or hkl, indices (a sort of zip-code for the planes).
Miller indices are simply a set of three numbers, hkl, which can take on any combination of three integer values between +∞ and −∞, e.g. (111), (-501) and (7-2-2). Each combination of hkl describes a unique set of planes filling the crystal and so hkl is often presented as a subscript to a property: e.g. dhkl which therefore means the d spacing between the planes defined by hkl. The Miller indices, hkl, also provide a useful means for visualising the planes. The convention is that if you have three axis, x, y, z, with three unit spacings, a, b, c, on each then one of these hkl planes can be visualised as the plane which intersects the x, y, z-axes at distances of a/h, b/k, c/l respectively
Q.2 What is a Space lattice, unit cell, Basis of a crystal? What is the coordination number and packing fraction?
Answer:
Basis:
A basis is a collection of atoms in particular fixed arrangement in space. We could have a basis of a single atom as well as a basis of a complicated but fixed arrangement of hundreds of atoms

Unit cell:
In general, the unit cell is chosen such that it is the smallest unit cell that reflects the symmetry of the structure. There are two distinct types of unit cell: primitive and non-primitive.
The coordination number (CN) is the number of nearest neighbours of a given particle in the crystal lattice. It determines the nature of the bonding in a crystal. The most common coordination numbers are 4, 6, 8, and 12.
Packing efficiency is the fraction of the unit cell that is occupied by particles. Closest packed spheres pack with a packing efficiency of 74%.
Spheres cannot be packed without creating some void space, but the amount of void space depends upon how well they are packed. Packing efficiency (PE) is that fraction of the unit cell volume that is actually occupied by particles, not void space., the packing efficiency of a unit cell is
Packing Efficiency | = |
× 100% | ||
|
|
| ||
|
|
| ||
PE | = |
× 100% |
a = the length of a side of the unit cell, so a3 is the volume of the unit cell.
Q.3. Define following:
- Atomic radius
- Co-ordination number.
- Unit cell.
- Lattice planes
Answer:
Lattice planes:
A set of parallel and equally spaced planes in a space lattice, which are formed with respect to the lattice points are called lattice planes.
Unit cell:
The unit cell is defined as the smallest geometric figure, the translational repetition of which in all over the three dimensions gives the actual crystal structure
(OR)
The unit cell may also be defined as the fundamental elementary pattern with minimum number of atoms, molecules (or) groups of molecules which represents the total characteristics of the crystal.
Atomic radius:
Atomic radius is defined as half of the distance between any two nearest neighbour atoms which have direct contact with each other, in a crystal of a pure element. It is interns of cube edge ‘a’. 24
Co-ordination number:
Co-ordination number is the number of nearest neighbouring atoms to a particular atom. (Or) Co-ordination number is the number of nearest neighbours directly surrounding a given atom
Q.4 Define Bragg’s law for X - ray diffraction and explain?
Answer:
Bragg’s Law can be derived using simple geometry by considering the distances travelled by two parallel X-rays reflecting from adjacent planes. The X-ray hitting the lower plane must travel the extra distance AB and BC. To remain in phase with the first X-ray, this distance must be a multiple of the wavelength thus:
nλ = AB+BC = 2AB (since the two triangles are identical)
The distance AB can be expressed in terms of the interplanar spacing (d) and incident angle (θ) because d is the hypotenuse of right triangle ZAB shown at right.
Sin(θ) = AB/d
Thus AB = d sin(θ)
Therefore:
nλ = 2 d sin(θ)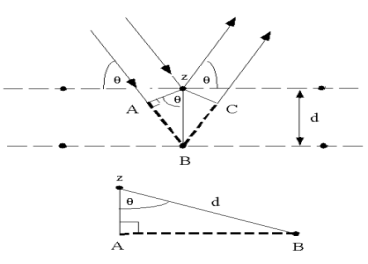
Reflection (signal) only occurs when conditions for constructive interference between the beams are met
These conditions are met when the difference in path length equals an integral number of wavelengths, n. The final equation is the
BRAGG’S LAW n λ = 2 d sin θ
Q.5 Define Atomic packing factor (or) Packing density (or) density of packing
Answer:
Atomic packing factor is defined as the ratio between the volume occupied by the total number of atoms per unit cell (v) to the total volume of the unit cell (V).

APF=v/V
Q.6 Obtain the relation between the cell edge and the atomic radius in the case of a boc unit cell.
Answer;
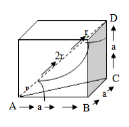
From the geometry, we can write
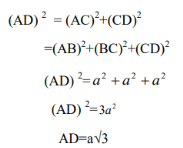
The diagonal of the cube AD=4R
Therefore
4r= a√3
r=a. √3/4
Q.7 What do you mean by interplanar spacing?
Answer:
The inter-planar spacing (dhkl) between crystallographic planes belonging to the same family (h,k,l) is denoted (dhkl) Distances between planes defined by the same set of Miller indices are unique for each material dhkl Inter-planar spacings can be measured by x-ray diffraction (Bragg’s Law) The lattice parameters a, b, c of a unit cell can then be calculated The relationship between d and the lattice parameters can be determined geometrically and depends on the crystal system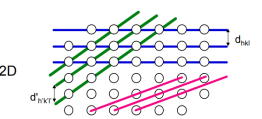
Q.8 Determine the total void volume (cm3 /mole) for gold (Au) at 27oC; make the hard-sphere approximation in your calculation, and use data provided in the periodic table.
Answer:
First determine the packing density for Au, which is FCC; then relate it to the molar volume given in the periodic table
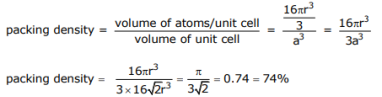
Void volume = 1 – packing density = 26%
From the packing density (74%) we recognize the void volume to be 26%. Given the molar volume as 10.3 cm3 /mole, the void volume is:
0.26x 10.3 cm3 / mole = 2.68 cm3 / mole
Q.9 what is Bragg’s diffractometer?
Answer:
Powder X-ray diffraction peak profile varies on instruments and conditions of measurements. It is necessary to take into account the effects of the instrument correctly, for precise evaluation of structures from powder diffraction data.
Since the instrumental function varies on which instrument is used, the instrumental function of an instrument may not be applicable to another instrument. Fortunately, there is a common design widely used for powder diffractometers. It is called as Bragg-Brentano design.
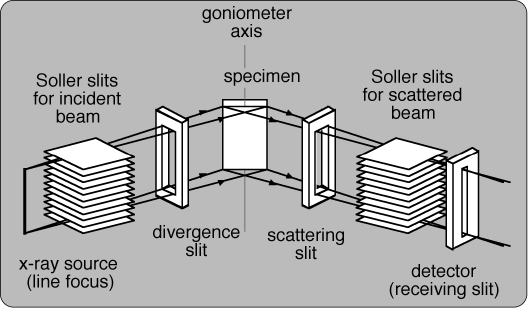
The configuration of the Bragg-Brentano diffractometer is very simple as illustrated above, but the lattice constants can be determined at the precision of five significant numbers by using this type of diffractometers. Since Bragg-Brentano diffractometers have been used for a long time.
Q.10 Describe three-dimensional crystal system and their Bravais lattices?
Answer:
If the atoms or molecules are uniquely arranged in crystalline solid or liquid, we call it as a crystal structure. A crystal possesses long range order and symmetry. The main property of crystal structure is its periodicity. This periodicity is due to the arrangement of atoms/molecules in the lattice points
Some common crystal structures you should know
 Simple Cubic |  Face Cantered Cubic |  Body Centered Cubic |  Hexagonal Close Packed |  Diamond |
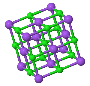 NaCl | 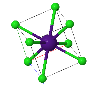 CsCl | 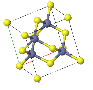 Zincblende | 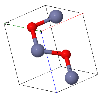 Wurtzite |  Perovskite |
Bravais Lattice
In solid state physics one usually encounters lattices which exhibit a discrete translational symmetry. If one considers for instance the vector space R3 this means that a translation of the whole lattice by any translation vector given by.
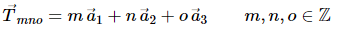
A lattice that can be characterised in this way is referred to as a Bravais lattice. All lattice points are equivalent, However, if there are lattice points with different environments they cannot form a Bravais lattice.