Unit - 4
Aromatic hydrocarbons
Q1) Define Huckel’s Rule?
A1) In organic chemistry, the aromatic qualities of any planar ring-shaped molecules are estimated by the Huckel’s Rule. The quantum mechanics was supported by the formulation of this rule which was initially solved by the German physical chemist and physicist Erich Armand Arthur Joseph Hickel in the year 1931.
The Hickel 4n + 2 Pi Electron Rule
The cyclic molecules that are ring shaped, follow Hickel rule when the total number of pi electrons belonging to the molecule can be equated to the formula ‘4n + 2’ where n is any integer with a positive value (including zero).
Examples of molecules following Hickel’s rule have only been established for values of ‘n’ ranging from zero to six. The total number of pi electrons in the benzene molecule depicted below can be found to be 6, obeying the 4n+2 𝛑 electron rule where n=1.
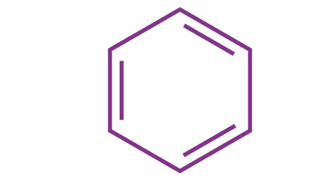
Therefore, the aromaticity of the molecule benzene is established as it obeys the Huckel rule.
This rule is also justified with the help of the Pariser-Parr-Pople method and the linear combination of atomic orbitals (LCAO) method.
The resonance energy or the localised electron cloud provide stability to the aromatic compounds. For a molecule to exhibit aromatic qualities, the following conditions must be met by it:
- There must be 4n + 2 𝛑 electrons present in a system of connected p orbitals (where the electrons are delocalized) belonging to the molecule.
- In order to meet the first condition, the molecule must have an approximately planar structure wherein the p orbitals are more or less parallel and have the ability to interact with each other.
- The molecule must have a cyclic structure and must have a ring of p orbitals which doesn’t have any sp3 hybridized atoms.
Other examples of aromatic compounds that comply with Huckel’s Rule include pyrrole, pyridine, and furan. All three of these examples have 6 pi electrons each, so the value of n for them would be one.
Q2) Define Aromatic hydrocarbons?
A2) The aromatic hydrocarbons are “unsaturated hydrocarbons which have one or more planar six-carbon rings called benzene rings, to which hydrogen atoms are attached”. Many aromatic hydrocarbons contain a benzene ring (also referred to as an aromatic ring). The benzene ring is stabilized by resonance and the pi electrons are delocalized in the ring structure
Aromatic Hydrocarbons are circularly structured organic compounds that contain sigma bonds along with delocalized pi electrons. They are also referred to as arenes or aryl hydrocarbons.
The aromatic hydrocarbons which do not contain a benzene ring are commonly referred to as heteroarenes. All of these heteroarenes obey Huckel’s rule (total number of pi electrons in a monocyclic ring = 4n + 2 where n is any positive integer or zero).
In these types of compounds, a minimum of one carbon is replaced by either nitrogen, oxygen, or sulphur. Common examples of heteroarenes include furan (contains oxygen) and pyridine (contains nitrogen).
Q3) Explain the properties of Aromatic Hydrocarbons?
A3) “The first compound that was categorized as an aromatic hydrocarbon was benzene”. It is also the most complex aryl hydrocarbon. Each carbon atom belonging to the benzene ring has two carbon-carbon sigma bonds, one carbon-hydrogen sigma bond, and one double bond with a neighbouring carbon in which the pi electron is delocalized.
This delocalization of pi electrons in the benzene molecule is represented by a circle inside the hexagon. The bond order of all carbon-carbon bonds in this molecule is considered to be 1.5 and this equivalency can be explained with the help of the resonance structures of benzene.
Some general properties of aromatic hydrocarbons have been listed below.
- These compounds exhibit aromaticity (additional stability granted by resonance)
- The ratio of carbon atoms to hydrogen atoms is relatively high in these types of molecules.
- When burnt, the aromatic hydrocarbons display a strong and sooty flame which is yellow.
- These compounds generally undergo electrophilic substitutions and nucleophilic aromatic substitution reactions.
The use of aromatic hydrocarbons is common in both biological and synthetic processes. Some numerous uses of aromatic hydrocarbons are listed below.
- The green pigment found in plants, more commonly known as chlorophyll, consists of aromatic hydrocarbons and is very important in the process of food production in plants.
- The nucleic acids and amino acids in the human body also consist of these aromatic hydrocarbons.
- Methylbenzene which is an aromatic hydrocarbon is used as a solvent in model glues
- Naphthalene is an important item in the production of mothballs
- For the synthesis of drugs, dyes, and explosives, an aryl hydrocarbon known as Phenanthrene is used
- Trinitrotoluene or TNT is a very important aromatic hydrocarbon which is widely used for explosive purposes.
- Plastic industry and petrochemical industries make use of aromatic hydrocarbons extensively.
Q4) How do you understand the Aromatic nature of Arenes?
A4) All arenes have general formula [CnH2n - 6y]. Where y is number of benzene rings and n is not less than 6.
Arenes are planar and cyclic. They undergo replacement rather than addition reactions.
Aromaticity or aromatic character: The characteristic behaviour of aromatic compounds is known as aromaticity. Aromaticity is because of extensive delocalisation of p-electrons in planar ring system. Huckel (1931) explained aromaticity on the basis of following rule.
For aromaticity the atom can be planar, cyclic system having delocalised (4n + 2) Π electrons where n is an integer equal to 0, 1, 2, 3,------.
Thus, the aromatic molecules have delocalised electron cloud of 2,6,10 or 14 p electrons.
For example: 4n +2 = 6; 4n = 4; n = 4/4 = 1
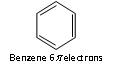 n = 1
n = 1
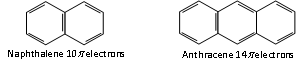
n= 2 n = 3
Q5) What do you understand by Cyclic Carbocations in Arenes?
A5) Carbocations, are compounds that possess trivalent positively charged carbon when we consider a stabilised carbocation like tert-butyl carbocation, this concept becomes true, but, if the carbocation moiety is contained in a cyclic, conjugated system having 4n+2 pi electrons, the carbocation may be stable enough even to isolate as a salt.
a) A particularly effective example of aromatic carbocation is the cycloheptarienyl (tropylium) carbocation, this cation is similar to benzene and cyclopentadienyl anion, it has a six-pi benzene electron system. Again, the electron count is properly obtained by counting 2 electrons per pi bond and zero electrons for a carbocation centre (vacant 2p AO) in any canonical structure.

b) This cation is isolated as a salt with many counter anions that also includes tetrafluoroborate anion. It is stable in aqueous solution.
c) An isolable aromatic carbocation can be prepared in the highly strained cyclopropenyl system. Note that in the case of a cyclopropenyl system there is only one BMO, so that the aromatic system contains 2 electrons (4n+2, with n = 0).

d) The circle mnemonic and the resulting display of MOs for the cyclopropenyl system is illustrated below:
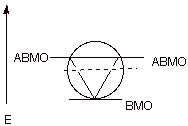
e) Stable aromatic anions and cations having 10, 14, and many higher electron count systems have also been prepared.
Carbanions and carbocations may also show aromatic stabilization. Some examples are:
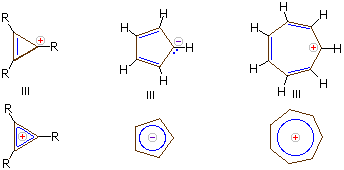
Considering its ring strain the three membered ring cation has 2π-electrons and is stable, Cyclopentadiene is as acidic as ethanol, reflecting the stability of its 6 π-electron conjugate bases. Salts of cycloheptatrienyl cation (tropylium ion) are stable in water solution, again reflecting the stability of these 6 π-electron cations.
Q6) Explain the Heterocyclic nature of compounds with examples?
A6) Heterocyclic compounds such as pyridine, fyran, thiophene and pyrrole are all aromatic since each one of them is planar and has cyclic system of 6n electron which is completely delocalised over the entire range
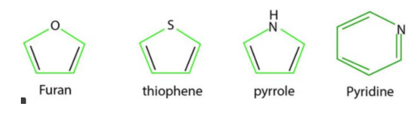
In Pyridine, each of the 5sp2 hybridised carbon atoms and the sp2 hybridised nitrogen atom has a p orbital perpendicular to the plane of the ring. Each of these atoms contributes one n electron thereby producing a single cyclic n-cloud containing 6n electrons. The lone pair of electrons on the nitrogen atom is present in a sp2 orbital which is being in the plane of the ring does not contribute towards the aromatic sextet
In Furan and Thiophene, one of the lone pairs of electrons on the sp2 hybridised heteroatom is present in a p orbital perpendicular to the plane of the ring, it contributes two n electrons while the other four p-orbitals of the two double bonds contribute one electron n each thereby the total to 6 electrons.
Pyrrole has only one lone pair which is present in a p-orbital perpendicular to the ring. Therefore , it contributes two n electrons while the fourp orbitals of the two double bond contribute one n electron thereby bringing the total to six electrons.
All the three five membered heterocycles have a single electron cloud containing 6 electrons and hence are aromatic in nature, this theory of six electrons explaining the aromatic character of cyclic compounds is called aromatic sextet theory and the six n electrons are called aromatic sextet
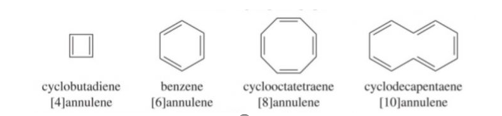
Annulenes: completely conjugated monocyclic polyenes containing an even number of carbon atoms are called annulenes, their general formula is (CH=CH) n where n=2,3,4…. Etc
Q7) Define an Electrophilic aromatic reaction?
A7) Electrophilic aromatic substitution reactions are organic reactions wherein an electrophile replaces an atom which is attached to an aromatic ring. Commonly, these reactions involve the replacement of a hydrogen atom belonging to a benzene ring with an electrophile.
The aromaticity of the aromatic system is preserved in an electrophilic aromatic substitution reaction. For example, when bromobenzene is formed from the reaction between benzene and bromine, the stability of the aromatic ring is not lost
A reaction in which one or more hydrogen of the benzene ring are replaced by other monovalent atoms or group is called a substitution reaction
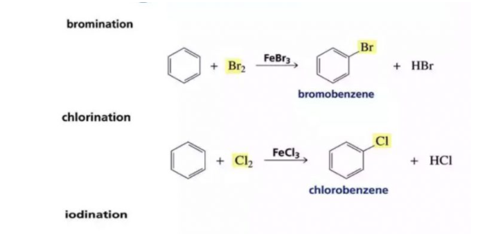
Q8) How does Halogenation take place in Arenes?
A8) Halogenation is a type of substitution reaction where hydrogen is replaced with bromine or chlorine.
- The process (chlorination or bromination) makes use of a Lewis acid, which takes a pair of electrons to form a permanent bond dipole in the Cl-Cl bond or the Br-Br bond.
- The Bromine or chlorine has a formal positive charge due to the dipole making the group electrophilic enough to easily overcome the activation energy which is generated due to the loss of aromaticity of the benzene ring.
- Halogenation: Benzene reacts with chlorine and bromine in the presence of Lewis acid as AlCl3, FeCl3, FeBr3 as catalyst and in the absence of light to form chlorobenzene and bromobenzene
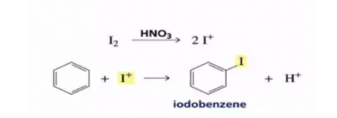
If excess of electrophilic reagent is used, two or more or even all the hydrogen atoms of benzene ring may be replaced by the electrophilic.
Benzene on treatment with excess of Cl2 in the presence of anhydrous AlCl3 in the dark yield hexachlorobenzene

The function of lewis is to carry the halogen to the aromatic hydrcarbons, In addition to iron and aluminium halides , iodine and iron filling have been usd as halogen carrier
Chlorobenzene is formed when benzene is treated with chlorine in presence of iron, under these conditions, iron forst reacts with chlorine to form ferric chloride which then act as a catalyst. Fluorinationof arenes is too vigorous to be of any practical use.
Q9) What reaction tales place in Arenes when a hydrogen is replaced with sulphonic acid?
A9) Sulfonation occurs when hydrogen is replaced with the help of sulfonic acid (SO3).
- This reaction is also quite similar to nitration where an electrophile is generated by protonation of SO3 with H2SO4.
- This helps to generate a strong electrophile. Once the item is obtained, the reaction follows the electrophilic aromatic substitution mechanism.
- Sulphonation: The process of replacement of a hydrogen atom of an arene by a sulphonic acid group (-SO3H) is called Sulphonation. It is usually carried out by treating an arene with fuming sulphuric acid or oleum or chlorosulphuric acid.

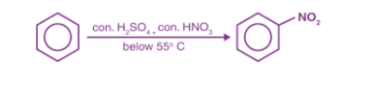
Q10) Define Nitration in Aromatic Hydrocarbons?
A10) Nitration reaction involves the replacement of a hydrogen with a nitro (NO2) group.
- Sulfuric acid (H2SO4) is used as a catalyst in this process.
- The acid is used to protonate the nitric acid which leads to the formation of a nitronium ion.
- The nitronium ion can then be processed as per the mechanism of electrophilic aromatic substitution reaction.
- The process of replacement of a hydrogen atom of an arene by the nitro(-NO2) is called Nitration, it is usually carried out by treating an arene with a mixture of conc HNO3 and conc H2SO4
Q11) Explain the Alkylation in Friedel crafts reactions?
A11) Friedel crafts alkylation: when benzene of its homologue is treated with an alkylhalide, in the presence of anhydrous aluminium chloride as catalyst, it forms an alkylbenzene.
Friedel-Crafts Alkylation involves the replacing of aromatic proton with an alkyl group, this is achieved by the electrophilic attack on the aromatic ring with the assistance of a carbocation. It is a method of generating alkylbenzenes using alkyl halides as reactants.
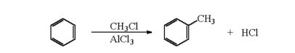
The Friedel-Crafts alkylation reaction proceeds via a three-step mechanism.
Step 1
Electrophilic carbonation is formed, when an alkyl halide reacts with The Lewis acid catalyst (AlCl3).
Step 2
Cyclohexadienyl cation is formed as an intermediate, when carbocation proceeds to attack the aromatic ring. The aromaticity of the arene is temporarily lost due to the breakage of the carbon-carbon double bond.
Step 3
The deprotonation of the intermediate leads to the reformation of the carbon-carbon double bond, restoring aromaticity to the compound. This proton goes on to form hydrochloric acid, regenerating the AlCl3 catalyst.
Q12) Define Friedel Craft Acylation?
A12)
a) Friedel Crafts acylation: On treatment with a carboxylic acid chloride or the anhydride in the presence of anhydrous aluminium chloride, benzene forms acylbenzene

The Friedel-Crafts acylation reaction includes the adding of the acyl group to the aromatic ring. This is achieved by the involvement of an acid chloride (R-(C=O)-Cl) and also a Lewis acid chloride catalyst like AlCl3. In the acylation reaction, the aromatic ring is changed into a ketone.
Mechanism
Friedel-Crafts acylation’s proceed through a four-step mechanism.
Step 1
The reaction takes place between the acyl halide and the Lewis acid catalyst (AlCl3), resulting in the formation of a complex, and the acyl halide loses a halide ion, forming an acylium ion which is stabilized by resonance.
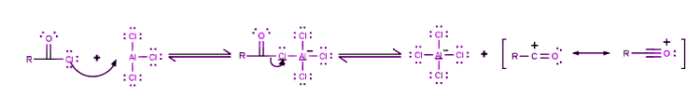
Step 2
The acylium ion (RCO+) performs an electrophilic attack on the aromatic ring, however the aromaticity is lost as the complex is being formed.
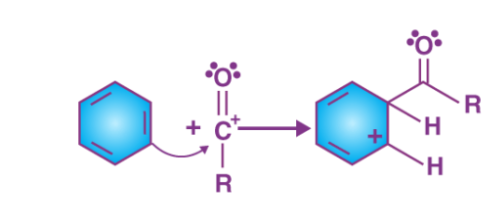
Step 3
The aromacity to the ring is restored as the intermediate complex is now deprotonated, the proton attaches itself to a chloride ion (from the complexed Lewis acid), forming HCl. The AlCl3 catalyst is now regenerated
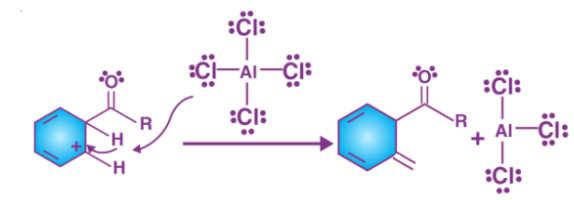

Step 4
The catalyst that is regenerated attacks the carbonyl oxygen, hence the liberation of ketone takes place by the addition of water to the products that were formed in step3. This step can be illustrated as follows.


Thus, at the end of Friedel-Crafts acylation the require acylbenzene is formed
Q13) Explain the steps involved in Electrophilic Aromatic Substitution Mechanism?
A13) Electrophilic Aromatic Substitution Mechanism
An electrophilic aromatic substitution consists of three main fundamental components:
- During the reaction, a new σ bond is formed from a C=C in the arene nucleophile.
- Proton is removed by the breaking of C-H σ bond.
- The C=C is reformed which restores the aromaticity.
As for the mechanism of the reaction, it usually includes two main steps.
Step 1
- The reaction begins by the electrophile attacking the pi electrons present in the aromatic benzene ring.
- This results in the formation of positively charged and delocalized cyclohexadienyl cation or a resonance-stabilized carbocation known as an arenium ion.
- This ion basically contains three resonance contributors. The electrophilic attacking the aromatic ring generally takes time and is a slow process.
- It is further endergonic and there is a presence of high activation energy due to the loss of aromaticity.
- Some main factors that are used to determine the attack of the electrophile are resonance, probability, and steric hindrance.
Step 2
- This step involves the deprotonation of the arenium ion by a weak base.
- The carbocation intermediate that is formed is attacked by a base that results in the loss of a proton.
- The electrons are then used to reform a pi bond and aromaticity is yet again restored.
- This is a very fast process and usually exergonic. An important thing to remember here is that the carbocation loses a proton as a result of the electrophile attacking the benzene ring.
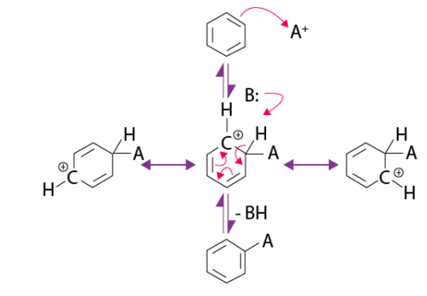
Q14) Write few uses of Aromatic Hydrocarbons?
A14) The use of aromatic hydrocarbons is common in both biological and synthetic processes. Some numerous uses of aromatic hydrocarbons are listed below.
- The green pigment found in plants, more commonly known as chlorophyll, consists of aromatic hydrocarbons and is very important in the process of food production in plants.
- The nucleic acids and amino acids in the human body also consist of these aromatic hydrocarbons.
- Methylbenzene which is an aromatic hydrocarbon is used as a solvent in model glues
- Naphthalene is an important item in the production of mothballs
- For the synthesis of drugs, dyes, and explosives, an aryl hydrocarbon known as Phenanthrene is used
- Trinitrotoluene or TNT is a very important aromatic hydrocarbon which is widely used for explosive purposes.
- Plastic industry and petrochemical industries make use of aromatic hydrocarbons extensively.
Q15) Define Arenes?
A15) Arenes are the benzene and its derivatives that refer to hydrocarbons own one or more benzene rings. The origin name is formed in the early organic development, because this class of compounds is almost always found in volatile, scented substances, such as: benzoic acid from benzoin gum, benzaldehyde obtained from bitter almond oil. But many of aromatic compounds are not fragrant, so today's arenes are compounds containing benzene ring. The most simple and most important arenes are benzene and its homologues toluene, xylene, ethylbenzene and so on.
Q16) Explain the use of Arenes in the Medicine Industry?
A16) Salicylic acid is one of the most famous arenes. In ancient times, salicylic acid has been not only used to relieve pain and fever, but also have anti-inflammatory effect. In modern medicine, methyl salicylate is also used to relieve joint and muscle pain; salicylic acid choline is widely used to treat oral ulcers. Salicylic acid is also a key exfoliating ingredient in many skin care products and is used in the treatment of acne, seborrheic dermatitis, psoriasis, corns and keratosis. Topical salicylic acid has antibacterial properties against the microbes, and its corrosion resistance is close to that of phenol
Q17) Explain the directing effects of the groups?
A17) In an electrophilic aromatic substitution reaction, the nature of the substituent which is present on the benzene ring affects the rate and regioselectivity (relative position) of the reaction. A substituent (-X) is said to be activating if the rate of electrophilic aromatic substitution of the substituted benzene (C6H5X) is faster than benzene. The electrophilic substitution rate of reaction is deactivated by the substituent, when the rate of the reaction of the substituted benzene (C6H5X) is slower than benzene. Relative rate of nitration:
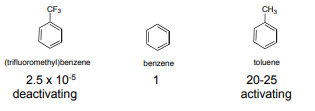

If the direction of the incoming electrophile is assisted by the substituent (-X) it is is said to be an ortho-para to itself. For example, if a substituent (-X) becomes a meta director if it assists the incoming electrophile to meta position to itself
Substituents can be divided into either electron-denoting or electron -withdrawing and can alter the electron density of the aromatic ring through:
1. Inductive effects: it is the ability of a substituent to donate or withdraw electron density through σ-bonds due to electronegativity differences and bond polarities of a functional group
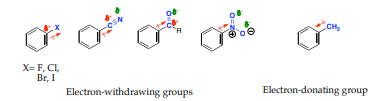
2. Resonance effects: It is the ability of a substituent to donate or withdraw electrons through non-bonding pairs of electrons or overlap π-bonds (conjugation).
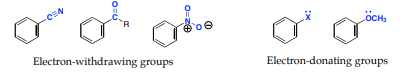
Q18) Explain coupling reactions in Arenes?
A18) In these types of reactions, the coupling of two fragments which have a radical nature is achieved with the help of a metal catalyst. When aromatic hydrocarbons undergo coupling reactions, the following type of bonds can be formed.
- Carbon-carbon bonds can be formed from the coupling reactions of arenes and products such as vinyl arenes, alkyl arenes, etc. are formed.
- The formation of carbon-oxygen bonds can occur in these reactions, forming aryloxy compounds.
- Carbon-nitrogen bonds can form in coupling reactions, giving products such as aniline.
An example of a coupling reaction involving aromatic hydrocarbons can be observed in the arylation of perfluorobenzenes, as illustrated below.
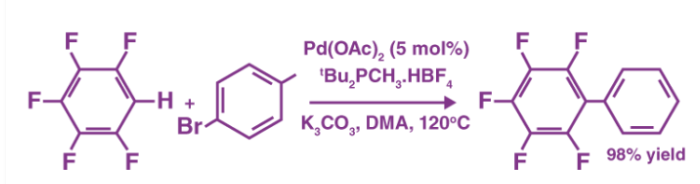
The catalyst used in this reaction is Palladium (II) acetate. It can also be noted that DMA is the abbreviation of Dimethylacetamide.
Q19) What are the kinds of reactions that take place in Aromatic hydrocarbons?
A19) These reactions involve the replacement of one substituent on the ring of an aromatic hydrocarbon, commonly a hydrogen atom, by a different substituent group.
The common types of aromatic substitution reactions include:
- Nucleophilic aromatic substitution reactions
- Electrophilic aromatic substitution reaction
- Radical nucleophilic aromatic substitution reactions
An example of an aromatic substitution reaction is the electrophilic substitution observed in the nitration reaction of salicylic acid.
Q20) Explain the use of benzene in industry?
Q21) The biggest use of benzene is to prepare the styrene, and polystyrene can be obtained by the polymerization of styrene. Polystyrene has excellent electrical properties, with well heat resistance and low price, and has become one of the most commonly used general-purpose plastics today. It is commonly used as shockproof packaging materials. Followed by hydrogenation of benzene into cyclohexane, cyclohexane is the raw materials to produce nylon. The use of nylon includes making fibers and engineering plastics, the fibers can be woven into a variety of fabrics and can also be made into the cords of aircraft and automobile tire. Industrial xylenes are a mixture of three xylene isomers (ortho, meta and para) and ethylbenzene. Among them, para-xylene can be made into terephthalic acid at high temperature, which is the main raw material for synthesizing polyester resin (polyester). Anthracene and naphthalene are polycyclic aromatic hydrocarbons, which are important raw materials for the production of synthetic dyes and pharmaceuticals.