Unit - 1
Chemical thermodynamics
Q1) Explain the Reversible and Irreversible reaction?
A1) Reversible reaction
Any changes which can be reversed or are a temporary conversion are known as reversible changes. The reactions which are reversible are called reversible reactions. In this reaction, one substance is modified into another form but a new compound is not formed. Processes such as melting, boiling, evaporation, freezing, condensation, dissolution are reversible changes. Few examples are melting of wax, freezing of ice, boiling water which evaporates as steam and condenses back to water.
Reactions are an interaction of two or more compounds called reactants to produce a product(s). In a reversible reaction, reactants and products formed are connected by a two-way arrow (⇌). This means reactants can be obtained back from the products.
Consider the reaction below,
A +B ⇌ C + D
Here, A and B are two reactants which react to give C and D. The two-headed arrow indicates that reaction is reversible and the reactants, A and B can be obtained from C and D.
Irreversible reaction
In contrast to reversible reaction, irreversible changes are permanent changes. Reactants react to form an entirely new compound and cannot be reversed. Heating, burning, mixing, powdering are few processes which cause irreversible changes. A common observable example is the cooking of raw egg which can’t be converted back to its original form. Ash obtained by the combustion of paper or any other substances is another example.
When a reaction is taking place in a unidirectional way such reactions are called irreversible reactions. In such reactions in a period of time reactants react completely to form a product. Here reaction is denoted by a one-way arrow (→).
For example,
A → B +C
Here, A is the reactant which is completely converted into products B and C which do not react to form A.
Q2) What is Reaction Enthalpy?
A2) Reaction enthalpy
During any chemical reaction, heat is either absorbed or given out. The heat exchange between the chemical reaction and its environment is reaction enthalpy (H). This cannot be measured directly. For this, there is a measurement of change in the temperature of a reaction over time to the final change in enthalpy denoted by ΔH.
From the value of ΔH, whether in a reaction heat is absorbed from the environment [endothermic] or is given off to the environment [exothermic] is determined. In general, ΔH is given as, ΔH = m×s×ΔT, where m is the mass of reactants, s is the specific heat of products, and ΔT is a change in temperature during the completion of the reaction.
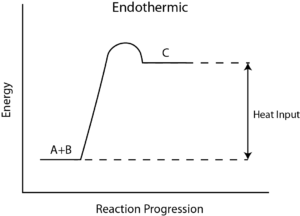
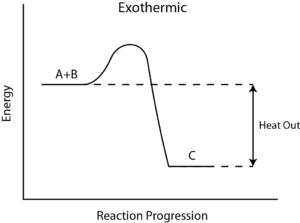
How to Calculate the Reaction Enthalpy?
To find enthalpy of a chemical reaction we need to follow the below mentioned steps.
- Determine products and reactants of the reaction
- Find total mass of reactants
- Determine the specific heat values of the products
- Find the difference in temperature between the start and end of a reaction
- Finally, multiply the mass of reactants by the heat values and that number, in turn, by the change in temperature
Finally, we will get the enthalpy of the chemical reaction.
Example of Enthalpy Calculation
Hydrogen reacts with oxygen to give water. The chemical reaction is,
2H2 + O2 → 2H2O
Reactants Products
- Step 1: Determining reactants and products – In this reaction, H2 and O2 are the reactants and H2O is the products.
- Step 2: Determination of the total mass of reactants – We can find total mass of reactants from the molar masses which can be formed in the periodic table
- 2H2 + O2 = 2H2O
2×[2g] 1×[32g] = 4g + 32g =36g
- Step 3: Determining specific heat of products – Every product has a specific heat value associated with it, which are constant values. Specific heat values carry the unit joule/gram°C.
2H2 + O2 = H2O
The specific heat of water is 4.2 joule/gram°C
- Step 4: Finding the difference in temperature after completion of reaction – For this, we have to subtract initial temperature (T1) of the reaction from the final temperature (T2) of the reaction, expressed in Kelvin.
ΔT = T1 – T2 (T1 = 185K and T2 = 95 K) = 185K – 95K
ΔT = -90K
In the above reaction, the reactants had more temperature, and after the completion of the reaction, the product got cooled off.
- Step 5: Use of formula ΔH = m×s×ΔT
ΔH = 36g×42 jg-1k-1 × (-90) K = -13608 joule
ΔH < 0
- Step 6: Determining whether this reaction gained or lost energy – ΔH determines whether the reaction gains or loses heat. The positive sign of ΔH means, the reaction gains heat and hence is endothermic. If the sign of ΔH is negative, the reaction is exothermic.
This makes logical sense since hot gases hydrogen (H2) and oxygen(O2) react together and let off heat to the environment. Then water [H2O] forms, indicating that it is an exothermic reaction.
Q3) What do you understand by Bond energy?
A3) It is a measure of a chemical bond strength, which means that it tells the possibility of a pair of atoms to remain bonded in the midst of energy perturbations. Alternatively, it can also be thought as the stability that is obtained when two atoms bond to each other as opposed to their unbounded states.
Bond energy and its determination
The Bond energy is determined by calculating the heat that is required to break one mole of molecules into their individual atoms, it denotes the average energy that is associated with breaking the individual bonds of a molecule. The higher the bond energy, the ‘stronger’ we say the bond is between the two atoms, and the distance between them (bond length) is smaller.
For instance, the HO-H bond in a molecule of water involves 493kJ/mol to break and generate the hydroxide ion (OH–). Further the breaking of the O-H bond in the hydroxide ion needs an additional of 424 kJ/mol. Therefore, the bond energy of the covalent O-H bonds in water is determined to be the average of the both the values, or 458.9 kJ/mol. These energy values (493 and 424 kJ/mol) are required to break successive O-H bonds in a water molecule and are generally called as ‘bond dissociation energies,’ and they are different from the bond energy. Therefore, the bond energy is the average of the bond dissociation energies in a molecule.
The exact properties of a specific kind of bond are determined in part by the nature of the other bonds in the molecule; for example, the energy and length of the C–H bond will vary depending on what other atoms are bonded to the carbon atom. Similarly, the C-H bond length can vary by as much as 4% between different molecules. For this reason, the values of bond energy and bond length are usually averages taken over a variety of compounds that contain a specific atom pair.
Q4) What is a Thermodynamic System Elaborate?
A4) Thermodynamics is a branch of physics that deals with the relation between other forms of energy and heat, in specific terms, it defines how the thermal energy gets converted to and from other forms of energy and how it affects matter.
Thermodynamic systems:
Thermodynamics in biology is the study of energy transfers that take place in molecules or in a collection of molecules. Generally, when dealing with Thermodynamics, a specific item or a collection of items that we are dealing with is called a system, while everything that's not included in the system, we’ve defined is called the surroundings.
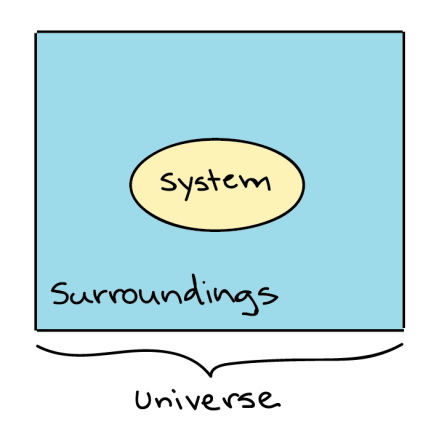
Generalized depiction of the system (a circle), the surroundings (a square surrounding the circle), and the universe (system + surroundings).
For example, if a pot of water is heated on the stove, the system included the stove, pot and water, while the surrounding could be the rest of the items in the kitchen, the decision of what to define as the system is arbitrary (up to the observer), and depending on what you wanted to study, we could equally well make just the water, or the entire house, part of the system. The system and the surroundings together make up the universe.
Q5) What are the different systems in Thermodynamics?
A5) There are three types of systems in thermodynamics: open, closed, and isolated.
- An open system is a system where the heat and matter are exchanged with the surrounding. The stovetop example would be an open system, because heat and water vapor can be lost to the air.
- A closed system, on the contrary, this system will exchange only energy with its surroundings, not matter, if the pot is tightly fitted with a lid would be a good example for closed system.
- An isolated system is one that cannot exchange either matter or energy with its surroundings. A perfect isolated system is hard to come by, but an insulated drink cooler with a lid is conceptually similar to a true isolated system. The items inside can exchange energy with each other, which is why the drinks get cold and the ice melts a little, but they exchange very little energy (heat) with the outside environment.
- The system that doesn’t exchange energy, or matter with the surroundings is called an isolated system. The Zeroth law of thermodynamics states that thermodynamic processes have no effect on the energy of the system.
Example: Reactants present in a thermos flask or an insulated vessel, where neither energy nor the matter is exchanged with the environment.
Q6) Differentiate between the Intensive and Extensive properties?
A6) Intensive and Extensive properties
Thermodynamic properties can be divided into 2 (two) general classes such as intensive and extensive properties.
An intensive property, is the system’s physical property that does not depend on the size or the amount of material in the system. In contrast the extensive property of a system is not dependent on the system size or the amount of the material. According to the definitions, density, pressure and temperature are intensive properties and volume, internal energy are extensive properties.
Symbols for representing properties: Extensive properties are symbolized by upper case (capital) letter such as V (volume), KE (kinetic energy), PE (potential energy), etc. Intensive properties are symbolized by lower case letters such as v (specific volume), ke (specific kinetic energy), e, u (specific internal energy), h (specific enthalpy), etc. Mole based properties are symbolized by lower case letters with overbars. For example, kinetic energy and molar specific potential energy respectively.
Exceptions: Temperature (intensive), mass (extensive), and number of moles (extensive). The use of symbols for temperature, mass and moles are traditional.
Exceptions:
Symbols for Exceptional Properties
Parameter | Property | Traditional symbol |
Temperature | Intensive property | T |
Mass | Extensive property | M |
Number of moles | Extensive property | N |
Specific extensive properties, i.e., extensive properties per unit mass are intensive properties. For example: specific volume, specific energy, density, etc.
Q7) What do you mean by Heat Capacity?
A7) The heat capacity of a substance can be defined as the amount of heat required to change its temperature by one degree.
Thermodynamics in mainly concerned with heat, earlier heat was considered as a measure of an invisible fluid, caloric and present in any matter. The ability of a substance to hold this fluid was earlier referred to as the heat capacity of the system, the meaning however of today is that heat capacity is energy in transit. The development in thermodynamics and dependence of heat transfer on temperature changed the definition of heat.
Modern thermodynamics defines heat as the measure of the total internal energy of a system. In order to quantify the heat energy associated with matter and its dependence on temperature, two properties were defined. These properties were named as specific heat capacity and heat capacity of the system.
Heat Capacity Formula
- Heat energy is the measure of the total internal energy of a system. Which includes the potential energy of the molecules and the total kinetic energy of the system and the potential energy of the molecules.
- It has been seen that the internal energy of a system can be changed by either supplying heat energy to it, or doing work on it.
- The internal energy of a system is found to increase with the increase in temperature. The increase in the internal energy depends on the amount of matter and the temperature differences. Etc.
- Therefore, heat capacity is defined as the amount of heat energy required to raise the temperature of a given quantity of matter by one degree Celsius.
- Heat capacity for a given matter depends on its size or quantity and hence it is an extensive property. The unit of heat capacity is joule per Kelvin or joule per degree Celsius.
Mathematically,
Q=CΔT
Where Q is the heat energy required to bring about a temperature change of ΔT and C is the heat capacity of the system under study.
Q7) Define Heat Energy?
A8) Internal Energy(U)
An energy form inherent in every system is the internal energy, which arises from the molecular state of motion of matter. The symbol U is used for the internal energy and the unit of measurement is the joules (J).
Internal energy U of a body or a system, that has well defined boundaries, refers to the total kinetic energy formed by the motion of molecules and the potential energy associated with the vibrational motion and electric energy of atoms within molecules. Internal energy also takes into account all the chemical bonds. From a microscopic point of view, the internal energy may be found in many different forms. For any material or repulsion between the individual molecules.
Internal energy is a state function of a system and is an extensive quantity.
Q8) What do you understand by Work in a thermodynamic system?
A9) In thermodynamics, work performed by a system is the energy transferred by the system to its surroundings. Work is a form of energy; however, the energy is in transit. Here the work includes the process that is completed by the system or on a system. In general, work is defined for mechanical systems as the action of a force on an object through a distance.
W = F.d
Where:
W = work (J)
F = force (N)
d = displacement (m)
A thermodynamic system in an equilibrium state possesses a state variable known as the internal energy(E). Between two systems the change in the internal energy is equal to the difference of the heat transfer into the system and the work done by the system.
The first law of thermodynamics states that the energy of the universe remains the same. Though it may be exchanged between the system and the surroundings, it can’t be created or destroyed. The law basically relates to the changes in energy states due to work and heat transfer. It redefines the conservation of energy concept.
Q9) Write a note on Enthalpy?
A10) Enthalpy is the measurement of energy in a thermodynamic system. The quantity of enthalpy equals the total content of heat of a system, equivalent to the system’s internal energy plus the product of volume and pressure.
In thermodynamics, the enthalpy is the measure of energy in a thermodynamic system. It is the thermodynamic quantity equivalent to the total heat content of a system
The Heat of Reaction (also known and Enthalpy of Reaction), occurs in the constant pressure, it is the change in the enthalpy of a chemical reaction.
Enthalpy is a state function and a thermodynamic unit for measurement. The enthalpy is used to calculate the amount of energy per mole that is either produced or released in the reaction. Since enthalpy is derived from pressure, volume, and internal energy, all of which are state functions.
When a process begins at constant pressure, the evolved heat (either absorbed or released) equals the change in enthalpy.
Enthalpy change is the sum of internal energy denoted by U and product of volume and Pressure, denoted by PV, expressed in the following manner.
H=U+PV
Enthalpy is also described as a state function completely based on state functions P, T and U. It is normally shown by the change in enthalpy (ΔH) of a process between the beginning and final states.
ΔH=ΔU+ΔV
If the pressure and temperature don’t change throughout the process and the task is limited to pressure and volume, the change in enthalpy is given by,
ΔH=ΔU+PΔV
The flow of heat (q) at constant pressure in a process equals the change in enthalpy based on the following equation,
ΔH=q
Any Enthalpy of a reaction is dependent on its physical conditions of the surroundings such as pressure, temperature etc. In order to specify the standard enthalpy of any reaction, the standard enthalpy of a reaction is calculated when the components that participate in the reaction are in standard form, the components include reactants and products.
Q10) How does an Adiabatic process for an ideal gas occur?
A11) Adiabatically when an ideal gas is compressed, (Q=0) its temperature generally increases: when we consider an adiabatic expansion process, the ideal gas does not work and the temperature decreases, a few adiabatic compressions examples include compression occur in the cylinder of a car, where the gas-air mixture take place so quickly that there is no time for the mixture to exchange heat with its environment.
The figure below shows the adiabatic process in the free expansion of a gas, that is confined by a membrane to one side of a two-compartment that is thermally insulated container. When the membrane is punctured, the gases rushes to the other side of the container and therefore expand freely, Because the gas expands “against a vacuum” (p=0), it does no work, and because the vessel is thermally insulated, the expansion is adiabatic.
With Q=0 and W=0W=0 in the first law, ΔEint=0, ΔEint=0, so
Eint i = Eint f for free gas expansion.
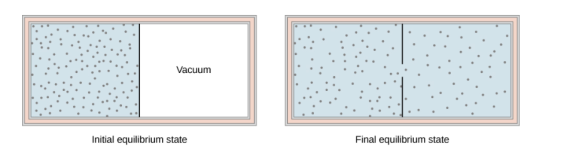
The gas in the left chamber expands freely into the right chamber when the membrane is punctured.
If the gas is ideal, the internal energy depends only on the temperature. Therefore, when an ideal gas expands freely, its temperature does not change.
Q11) Explain the adiabatic process for reversible reaction?
A12) an Adiabatic process no heat enters or leaves the system, therefore, for a reversible adiabatic process the first law takes the form
DU = − PdV
CV = (∂U/∂T)V (when the, molar heat capacity is taken into account)
But the internal energy of an ideal gas depends only on the temperature and is independent of the volume (because there are no intermolecular forces), and so, for an ideal gas,
CV = dU/dT, and so we have dU = CVdT.
Thus, for a reversible adiabatic process and an ideal gas,
CVdT = −PdV. (The minus sign shows that as V increases, T decreases, as expected.) But for a mole of an ideal gas,
PV = RT = (CP − CV)T, or P = (CP − CV)T/V.
Therefore
CvdT=−(CP−CV) TdV/V
Separate the variables and write γ for CP/CV:

Integrate:
TVγ−1= constant.
This shows how temperature and volume of an ideal gas vary during a reversible adiabatic expansion or compression. If the gas expands, the temperature goes down. If the gas is compressed, it becomes hot. Of course, the pressure varies also, and the ideal gas conforms to the equation
PV/T = constant. On elimination of T, we obtain
PVγ= constant
On elimination of V we obtain

Q12) What are the factors that affect the size of Hydration enthalpy?
A 13) Hydration enthalpy is a measure of the energy released when attractions are set up between positive or negative ions and water molecules.
- When ions are positive the attractions are loosened between the slightly negative oxygen atoms in the water molecules and the positive ions, or they may be covalent bond formation.
- When ions are negative the hydrogen bonds are formed between the lone pair of electrons on the negative ions and the slightly positive hydrogens in water molecules.
The size of the hydration enthalpy is governed by the amount of attraction between the ions and the water molecules.
- As we go down the periodic table the smallest lithium ion has the highest hydration enthalpy in group 1, the attractions are stronger when the ions are smaller, and the smallest fluoride ion has the highest hydration in group 7 The attractions are stronger the smaller the ion. In both groups, hydration enthalpy falls as the ions get bigger.
- The more highly charged the ion, the stronger is the attraction. For example, the hydration enthalpies of Group 2 ions (like Mg2+) are much higher than those of Group 1 ions (like Na+).
Estimating enthalpies of solution from lattice enthalpies and hydration enthalpies
The hydration enthalpies for calcium and chloride ions are given by the equations:
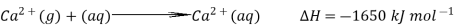
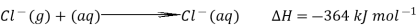
The following cycle is for calcium chloride, and includes a lattice dissociation enthalpy of +2258 kJ mol-1. We have to use double the hydration enthalpy of the chloride ion because we are hydrating 2 moles of chloride ions. Make sure you understand exactly how the cycle works.
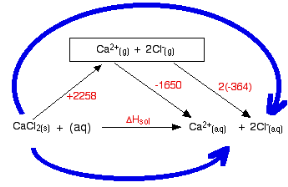
So
ΔHsol = +2258 - 1650 + 2(-364)
ΔHsol = -120 kJ mol-1
As the case above the negative hydration enthalpies are found to be more than the positive lattice dissociation enthalpy, depending on the size of the lattice enthalpy and the hydration enthalpy the enthalpy of solution corresponds to being positive or negative.
Q13) Describe the Enthalpies of ions and molecules?
A14) The enthalpy change with respect to a solution is the enthalpy change where 1 mole of an ionic substance dissolves in water to form a solution of indefinite dilution. The enthalpy of a solution is either negative or positive, in other words, some ionic substances dissolved endothermically (for example, NaCl); others dissolve exothermically (for example NaOH).
An infinitely solution that is dilute in one where there is an infinitely dilute solution is one where there is an adequately excess amount of water such that adding any further does not involve any heat to be absorbed or evolved. Therefore when 1 mole of sodium chloride crystals are dissolved in an excess of water, the enthalpy change of solution is found to be +3.9 kJ mol-1. The change is slightly endothermic, and so the temperature of the solution will be slightly lower than that of the original water.
When a substance dissolve why is it that heat is sometimes dissolved or absorbed. An imaginary process can be put forward the crystal lattice is first broken up into its separate gaseous ions and then those ions have water molecules wrapped around them. That is how they exist in the final solution.
- The heat energy needed to break up 1 mole of the crystal lattice is called the Lattice dissociation enthalpy
- The heat that is released when new bonds are formed between the ions and water molecules is called hydration enthalpy of the ion. The hydration enthalpy is the enthalpy change when 1 mole of gaseous ions dissolve in sufficient water to give an infinitely dilute solution. Hydration enthalpies are always negative.
Q14) Define Standard Enthalpy of combustion and its applications?
A15)
Standard enthalpy of combustion is defined as the enthalpy change when one mole of a compound is completely burnt in oxygen with all the reactants and products in their standard state under standard conditions (298K and 1 bar pressure). For example:
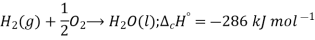
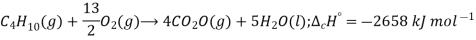
Applications
1. Calculation of heat of formation:
With respect to organic compounds, the heat of combustion is estimated easily, they are used to calculate the heat of formation of other compounds.
2) Calculation of calorific value of food and fuels:
The enthalpy of combustion is useful in calculating, the calorific value the calorific value is defined as the amount of heat produced in calories (or joules) when one gram of the substance is completely burnt. The SI unit of calorific value is J kg−1. However, it is usually expressed in cal g-1
Q15) What is Bond Dissociation energy?
A16) Bond dissociation energy refers to the amount of energy that is needed to break a chemical bond homolytic ally. A homolytic process results in formation of a radical species. Shorthand notation for this energy is BDE, D0, or DH°. Bond dissociation energy often useful in measuring the strength present in chemical bond and also compare different bonds. Note the enthalpy change is temperature dependent. Typical units of bond dissociation energy are kJ/mol or kcal/mol. Bond dissociation energy may be measured experimentally using spectrometry, calorimetry, and electrochemical methods.
Bond Dissociation Energy
- Bond dissociation energy is the energy required to break a chemical bond.
- The energy is a means of quantifying the strength of a chemical bond.
- Bond dissociation energy equals bond energy only for diatomic molecules.
- The strongest bond dissociation energy is for the Si-F bond. The weakest energy is for a covalent bond and is comparable to the strength of intermolecular forces.
Q16) Differentiate between Bond energy and Bond Dissociation energy?
A17) Bond dissociation energy is only equal to bond energy for diatomic molecules. This is because the bond dissociation energy is the energy of a single chemical bond, while bond energy is the average value for all the bond dissociation energies of all bonds of a certain type within a molecule.
For example, consider removing successive hydrogen atoms from a methane molecule. The first bond dissociation energy is 105 kcal/mol, second is 110 kcal/mol, third is 101 kcal/mol, and final is 81 kcal/mol. So, the bond energy is the average of the bond dissociation energies, or 99 kcal/mol. In fact, the bond energy doesn't equal the bond dissociation energy for any of the C-H bonds in the methane molecule!
Q17) Define Resonance energy?
A18) The difference in energy between the actual molecule and the canonical form of the lowest energy is called the resonance energy, the resonance energy of a molecule is expressed in Kcal/mole or kJ/mole
The resonance energy is directly proportional to the stability of a molecule, so the stability of a molecule increases with increasing its resonance energy. For example, the resonance energy of benzene is 36kcal/mole and the resonance energy of pyridine is 28kcal/mole, this indicates that benzene is more stable than pyridine.
Q18) Describe the effect of temperature on the enthalpy of reactions?
A19) In a chemical and physical process, the heat change depends on the temperature at which it takes place. This dependence on temperature is mathematically expressed in the form of what is known as Kirchhoff equation after G. R. Kirchhoff (1858) who first developed this equation. The equation may easily be derived with the help of the first law of thermodynamics.
Kirchhoff’s Equation is equality, it expresses the dependence of temperature of the thermal quantities that are linked with a chemical reaction, through the difference in heat capacities between the products and reactants. The same reaction, when carried at dissimilar temperatures, the enthalpies of reaction are also different.
Kirchhoff equation relates the heat of reaction with the definite heats of a structure before and after the reaction. Kirchhoff equation is represented as dQ/dt = C – C’
Where, Q is the heat energy evolved throughout the procedure at temperature ‘t’ without modifying in volume and C is the specific heats of the reactants and C’ is the specific heats of products.
A temperature transform occurs when the temperature is increased or decreased by the flow of heat. This shifts the chemical equilibrium toward the products or reactants, which can be resolute by studying the reaction and deciding whether it is endothermic or exothermic. The equation which indicates Effect of temperature on the heat of reaction is known as Kirchhoff’s equation.
Q19) Define Molar heat capacity?
A20) Definition of Molar heat capacity
The total amount of energy in the form of heat needed to increase the temperature of 1 mole of any substance by 1 unit is called molar heat capacity(C) of that substance. It also depends greatly on the nature, size and composition of a substance in a system.
q=n C ΔT
q is the heat supplied or needed to bring about a change in temperature(ΔT) in 1 mole of any given substance.
n is the amount of moles; the constant C is known as the molar heat capacity of the body of the given substance.
Cp: in a system, Cp is the amount of heat energy released or absorbed by a unit mass of the substance with the change in temperature at a constant pressure.in other words, under constant pressure it is the heat energy transfer between a system and the surroundings. So, Cp represents the molar heat capacity, C when pressure is constant. The change in temperature will always cause a change in the enthalpy of the system.
Enthalpy (ΔH) is the heat energy absorbed or released by the system, furthermore enthalpy change occurs during the change of phase or state of a substance.
For example, when a solid change to its liquid form (i.e., the change from ice to water) enthalpy change is called the heat of fusion. When a liquid change to its gaseous form (i.e., the change from water-to-water vapour) the enthalpy change is called the heat of vaporisation.