Unit – 4
Solutions and Colligative Properties
Q1) What are Colligative Properties?
A1) Colligative properties
Colligative properties, is a property of a solution that depends on the ratio between the total number of solute particles present in the solution and the total number of solvent particles. The Colligative properties are not dependent on the chemical nature of the solution’s components. Thus, these properties are linked to several quantities that express the concentration of a solution., such as molarity, normality, and molality. The four colligative properties that can be exhibited by a solution are:
- Boiling point elevation
- Freezing point depression
- Relative lowering of vapour pressure
- Osmotic pressure
The word “colligative” is taken from the Latin word has been adapted or taken from the Latin word “colligatus” which means to “bound together”. When a solution is defined, the colligative properties help to analyse how the properties of solution is linked to the concentration of solute in a solution.
Non-volatile solute that are dilute exhibit some properties that depend only on the number of solute particles present in the solution and not on the solute present in the solution. These properties are called colligative properties. Such properties are seen mostly in dilute solutions.
We can further consider colligative properties as those properties that are obtained by the dissolution of a non-volatile solute in a volatile solvent. Generally, the solvent properties are changed by the solute where its particles remove some of the solvent molecules in the liquid phase. This also results in the reduction of the concentration of the solvent.
Meanwhile, when we talk about the given solute-solvent mass ratio, colligative properties are said to be inversely proportional to the solute molar mass.
Colligative Properties Examples
We can observe the colligative properties of solutions by going through the following examples. If we add a pinch of salt to a glass full of water its freezing temperature is lowered considerably than the normal temperature. Alternatively, its boiling temperature is also increased and the solution will have a lower vapour pressure. There are changes in its osmotic pressure as well.
Similarly, if we add alcohol to water, the solution’s freezing point goes down below the normal temperature that is observed for either pure water or alcohol.
Q2) What do you understand by Osmotic pressure?
A2) Osmotic Pressure
It is the pressure that is required to prevent water from diffusing through a membrane by osmosis. Osmotic pressure is estimated by the concentration of the solute. Water diffuses to the area of higher concentration from the area of lower concentration, this continues until the concertation is uniform throughout (when concentrations of the substances in both areas are different)
Osmotic pressure can be calculated using the equation:
Π=MRT
Where Π denotes the osmotic pressure,
M is the molar concentration of the solute,
R is the gas constant,
T is the temperature
Q3) Explain an experiment to determine the Osmotic Pressure?
A3) Abbe Nollet (1748) was the first person to notice the phenomenon of osmosis and made measurements of the osmotic pressure. He chose pigs bladder as the semi permeable membrane, however the membrane failed to meet the requirements as the results were semi quantitative and the pig’s bladder was not a very good semi-permeable membrane. It was the botanist Pfeffer, he was successful in making a good semi permeable membrane and succeeded in making measurements for osmotic pressure. The Pfeffer Cell Apparatus, invented by Wilhelm Pfeffer in 1877, the instrument measured the minimum pressure that was required to stop a pure solvent from passing into a solution across a semipermeable membrane that is called the osmotic pressure.
The osmotic pressure of a solution at a particular temperature is defined as the additional hydrostatic pressure that is required that builds up when the solution is separated from the solvent by a semipermeable membrane. It is denoted by p.

Fig: The Pfeffer’s apparatus for determination of osmotic pressure
Pfeffer’s method: he developed a technique by depositing gelatinous copper ferrocyanide in the pores of an earthen pot, this earthen pot was used as the semipermeable membrane. Osmosis is mainly a diffusion of a solution through a semipermeable membrane. His apparatus for the measurement of osmotic pressure is shown in Figure. The porous pot, that has deposits of copper ferrocyanide in its pores is connected to a manometer. The pot s filled with the solution to be examined and this is placed in a vessel that consists of the solvent. The entire set up is paled in a thermostat and maintained this way till equilibrium is reached. At equilibrium, the difference in the heights of mercury columns in the two tubes gave the osmotic pressure. Osmotic pressure is the excess pressure that should be applied to a given solution in order to increase its vapor pressure until it becomes equal to that of the solution. The osmotic pressure of a solution is the pressure difference needed to stop the flow of solvent across a semipermeable membrane.
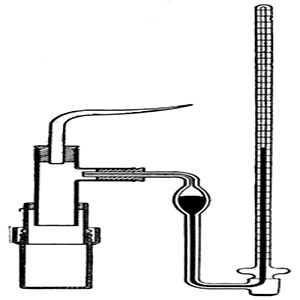
Fig: Pfeffer‘s apparatus for measurement of osmotic pressure
The osmotic pressure of a particular solution is proportional to the molar concentration of the solute particles present in the solution. This method is slow as it may take few days for equilibrium to be reached, also if the pressure is too high the membrane may break and it cannot be used again. When a semipermeable membrane (animal bladders, skins of fruits and vegetables) separates a solution from a solvent, then only solvent molecules are competent to pass through the membrane. Pfeffer’s method was improved by Berkeley and Hartley.
Q4) What is Van’t Hoff Factor?
A4) Van't Hoff factor
The Van’t Hoff factor offers insight on the effect of solutes on the colligative properties of solutions. It is denoted by the symbol ‘i’. The Van’t Hoff factor is defined as the ratio of the particle’s concentration formed when a substance is dissolved to the substance concentration by mass.
The degree to which a substance dissociates or associates in a solution is described by the Van’t Hoff factor. For example, the value of i is generally 1 when the non-electrolytic substance is dissolved in water, however when an ionic compound is dissolved in water the value of i is equal to the total number of ions present in one formula unit of the substance.
For example, the Van’t Hoff factor of CaCl2 is ideally 3, as CaCl2 dissociated into one Ca2+ ion and two Cl– ions. Sometimes these ions associate with each other causing a decrease in the total number of particles in the solution.
This factor is named after the Dutch physical chemist Jacobus Henricus Van’t Hoff, who won the first Nobel Prize in chemistry. It is important to note that the measured value of the Van’t Hoff factor for electrolytic solutions is generally lower than the predicted value (due to the pairing of ions). The greater the charge on the ions, the greater the deviation.
Van’t Hoff explained that when solutes are dissolved in a solvent they dissociate into ions. Since colligative properties depend only on the number of solute particles, the dissociation of solute molecules into ions results in an increase in the number of particles and hence affects the colligative properties.
Q5) Write a note on Vapour Pressure?
A5) Vapour Pressure
Vapour pressure is a property of liquid which related to evaporation. In other words, it is the pressure that is exerted by a vapour in equilibrium with its phases either as solid or liquid at a given temperature and in a closed system.
In simple terms, we can define vapour pressure as a measure of the tendency of a material to change its state to solid/liquid to gas or vapour when the temperature increases. It indicates the rate of evaporation of liquid. It is also closely related to the tendency of particles to escape from the liquid or solid-state.
Relation Between Vapour Pressure and Temperature
The substance with a high vapour pressure at room temperature is called a volatile substance. The temperature at which the vapour pressure at the surface of a liquid is equal to the pressure exerted by surrounding is denoted as the boiling point of that substance.
As the temperature of a liquid substance increases, the molecules’ kinetic energy also increases. Thus, the number of molecules transitioning into a vapour also increases and thereby increasing the vapour pressure of the liquid.
Simply say, with an increase in the temperature, the vapour pressure becomes sufficient to overcome atmospheric pressure and lift the liquid to form vapour bubbles inside the bulk of a substance. Volatile components such as gases and solvents are characterised by high vapour pressure can be transported with winds and components with low vapour pressure are transported with soil, sediment etc.
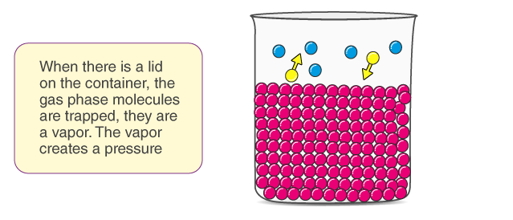
Fig: Pictorial Representation of Concept of Vapour Pressure
The vapour pressure of any substance increases nonlinearly with temperature. As moving to height, atmospheric pressure decreases because air is less dense at higher altitude. Because the atmospheric pressure is low then the vapour pressure of liquid needs to be lower to reach the boiling point. Therefore, low heat is required to make vapour pressure equal to atmospheric pressure.
The concept of vapour pressure can be understood by this example, when a closed container is taken and half filled with water. The container is then heated and evaporation takes place, the water in the container escapes in the form of vapour, some of the molecules will be converted to vapour during evaporation. As a result, the amount of liquid in the container decreases and the amount of vapour increases. The evaporated gas molecules will randomly move along the empty space in the container, as a result of this random movement, some of the molecules will come in contact with the uppermost layer of the container and begin to condense.
In the beginning the rate of evaporation will not be equal to the rate of condensation, after sometime this equilibrium will be reached. At that equilibrium, the rate of evaporation is equal to the rate of condensation.
As time changes the number of molecules in a gaseous state increases while the rate of condensation also increases. At last, it reaches a stage where the rate of evaporation becomes equal to the rate of condensation. This stage is known as the equilibrium stage. At this point, the pressure exerted by the molecules on the wall is known as the vapour pressure of the liquid.
Water Vapour Pressure
When we talk about the vapour pressure of water, it is nothing but the pressure that the water vapour experiences when it is in thermodynamic equilibrium. It will be in its condensed state. Generally, when the pressure is high water condenses.
Vapour Pressure Units
Vapour pressure can only be calculated in a closed container. Vapour pressure is usually measured in standard units of pressure. The SI unit is the pascal (Pa) where one pascal is equal to one newton per square meter (N·m−2 or kg·m−1·s−2).
Factors Affecting Vapour Pressure
There are four factors on which vapour pressure depends. They are:
Nature Of Liquid
Nature of liquid is explained on the basis of its intermolecular forces. That is, as the magnitude of the intermolecular forces increases vapour pressure will decrease.
Effect Of Temperature
As the temperature of the liquid increases the kinetic energy associated with the liquid also increases. And due to this increase in kinetic energy the escaping tendency of molecule increases and hence vapour pressure increases. So, we can conclude that vapour pressure is directly proportional to temperature.
Concentration Of Solute
The presence of solute in the liquid will decrease the vapour pressure. And this fall in vapour pressure also varies with the concentration of solute.
Vapour Pressure Is Independent Of
Humidity
Temperature is the only property that affects the vapour pressure for a certain amount of water vapour in the air. Humidity will affect only if all the other variables are constant. So don’t be confused between the effect of temperature and humidity.
Volume
Vapour pressure will not be affected by the volume of the container. As we know that liquid in the container will be in equilibrium with the vapour. When the volume is changed say decreased, then some of the vapour in the container turns into a liquid state. And if the volume increases some of the liquid will change into its vapour state.
Surface Area
Normally vapour pressure is independent of surface area. Change in surface area will not affect the vapour pressure of a substance.
Q6) Define the Relative Lowering of Vapour pressure?
A6) Raoult's Law of lowering of vapour pressure
Raoult’s law has been named after François-Marie Raoult, a French chemist who while conducting an experiment found out that when substances were mixed in a solution, the vapour pressure of the solution decreased simultaneously. Raoult’s law was established in the year 1887 and is also considered as the law of thermodynamics.
Raoult’s law states that a solvent’s partial vapour pressure in a solution (or mixture) is equal or identical to the vapour pressure of the pure solvent multiplied by its mole fraction in the solution.
Mathematically, Raoult’s law equation is written as;
Psolution = ΧsolventP0solvent
Where,
Psolution = vapour pressure of the solution
Χsolvent = mole fraction of the solvent
P0solvent = vapour pressure of the pure solvent
Relative Lowering of vapour pressure
The ratio of the lowering of vapour pressure of a solution and the vapour pressure of the pure component is known as relative lowering of vapour pressure.
The vapour pressure of a liquid is the pressure applied by its gaseous phase where condensation and vaporization are in equilibrium, when a non-volatile substance is dissolved in a volatile liquid it results in lowering of the liquid’s vapour pressure.
This concept can be explained by understanding the effect of non-volatile solute molecules on the liquid’s tendency of vaporisation., for vaporisation to take place the solvent molecules must be present at the surface of the solution, the rate of vaporisation is reduced by the presence of non-volatile solutes, as they decrease the surface area available to the solute molecules.
The net result is that the vaporisation condensation equilibrium is achieved with fewer solvent molecules in the vapour phase, the presence of non-volatile solutes lowers the vapour pressure of a solution by impeding the evaporation of solvent molecules.
Raoult’s law describes the relationship between the vapour pressures of solution components and the concentrations of the components.
The partial pressure exerted by any component of an ideal solution is equal to the vapour of the pure component multiplied by its mole fraction in the solution.
If A is the solvent and B is solute we can write

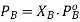
PA is the partial pressure exerte by component A in the solution , POA is the vapour pressure of Pure A and XA is the mole fraction in the solution.
B is a non volatile substance in one whose vapour pressure is negligible containing only non volatile solutes is due only to the solvent.
Since the solvent is the only volatile component of this solution, its vapour pressure may be computed as per Raoult’s law.
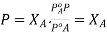 subtracting 1
subtracting 1
From each side we get 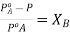 . This is expression for RLVP
. This is expression for RLVP
Since PB is zero
Hence we can say P is less than 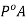 and we can write the lowering of vapour pressure as
and we can write the lowering of vapour pressure as
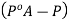
And the relative lowering can be written as
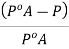
Q7) How is the Molar Mass determined from relative lowering of vapour pressure?
A7) Determination of molar mass weights from relative lowering of vapour pressure
The molar mass of a non-volatile solute can be determined by measuring the relative lowering of vapour pressure, for this a known mass of the solute is dissolved in a known solvent. The relative lowering of vapour pressure is measured experimentally.
According to Raoult’s law the relative lowering of vapor pressure is,
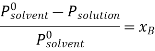
Let wAand wB be the weights of the solvent and solute respectively and their corresponding molar masses are MA and MB, then the mole fraction of the solute xB is
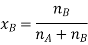
Here, nA & nB are the moles of the solvent and the solute respectively. For dilute solutions nA>>nB. Hence nA +nB ≈ nA. Now
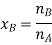
Number of moles of solvent and the solute are,
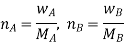
Therefore 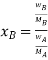
Thus Relative lowering of vapour pressure = 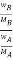
Relative lowering of vapour pressure
From the equation the molar mass of the solute (MB) can be calculated using the known the values of wA, wB, MA and the measured relative lowering of vapour pressure.
Q8) What do you understand by Elevation of boiling point?
A8) A non-volatile solute is added resulting in the lowering of vapour pressure through Raoul’s law, then the temperature must be raised to restore the vapour pressure to a value that corresponds to the pure solvent. The temperature at which vapour pressure is 1 atm will be higher than the normal boiling point by a known specific amount called the boiling point elevation. If addition of a non-volatile solute lowers the vapor pressure of the solution via Raoult's law, then it follows that the temperature must be raised to restore the vapor pressure to the value corresponding to the pure solvent. In particular, the temperature at which the vapor pressure is 1 atm will be higher than the normal boiling point by an amount known as the boiling point elevation. The precise relationship between the boiling point of the solution and the solvent molar fraction is very complicated, but for dilute solutions the elevation of the boiling point is directly proportional to the molal concentration of the solute:

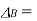 Boiling point elevation (K)
Boiling point elevation (K)
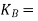 molal boiling point elevation constant
molal boiling point elevation constant 
The boiling point of a liquid is the temperature at which the vapour pressure is equal to atmospheric pressure. The addition of non-volatile liquid to a pure solvent, there is a decrease in the vapour pressure of the solution, to make this vapour pressure equal to atmospheric pressure, the temperature of the solution has to be increased. The difference in the boiling point of the solution and the boiling point of the pure solvent is termed as elevation in boiling point.
If T0b is the boiling point of the pure solvent and Tb is the boiling point of the solution then elevation in boiling point is given as
∆Tb =T0b-Tb
Experimental results show that there is a relation between elevation in boiling point and molality ‘m’ of the solute present in solution
∆Tb ∝ m
∆Tb = kb m
Where,
Kb = molal elevation constant
Substituting the value of ‘m’ in the above relation we get
∆Tb = 1000 x kb x m2 / M2 x m1
Where,
m2 = mass of solvent in g
M1 = mass of solvent in kg
M2 = molar mass of solute
Q9) Elaborate on the Freezing Point Depression?
A9) The freezing point of a substance is the temperature at which the solid and liquid forms can coexist indefinitely meaning they are in equilibrium that is, they are in equilibrium. In the presence of these conditions the molecules pass between the two phases at an equal rate, as their escaping tendencies from the two phases are similar.
For example, a liquid solvent and its solid like water and ice are at equilibrium given in (1 below), on addition of non-volatile solute (such as salt, sugar, or automotive antifreeze liquid) to the water. This will have the effect of reducing the mole fraction of H2O molecules in the liquid phase, and thus reduce the tendency of these molecules to escape from it, not only into the vapor phase (as we saw above), but also into the solid (ice) phase. This will have no effect on the rate at which H2O molecules escape from the ice into the water phase, so the system will no longer be in equilibrium and the ice will begin to melt 2.
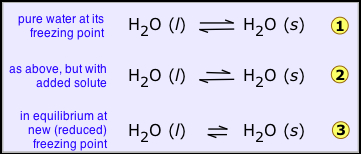
If the solid is prevented from melting the tendency of the molecules to escape, from the solid should be reduced, and this can be only achieved by reducing the temperature., this helps to lower the tendency of molecules to escape from both the phases but it affects those in the solid more than those in the liquid, so we eventually reach the new, lower freezing point where the two quantities are again in exact balance and both phases can coexist 3.
If vapour pressure is taken into consideration, then the vapour pressure of the solid and liquid should have the same freezing point. Dilution of the liquid (the solvent) by the non-volatile solute results in the decrease of vapour pressure of the solvent by the Raoult’s law, therefore reducing the temperature of the vapour pressure of the liquid frozen forms of the solution will be equal. As with boiling point elevation, in dilute solutions there is a simple linear relation between the freezing point depression and the molality of the solute:
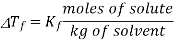
Note that Kf values are all negative!
The freezing point of a substance is defined as the temperature at which the vapour pressure of its liquid is equal to the vapour of the corresponding solid. According to Raoult’s law when a non-volatile solid is added to the solvent its vapour pressure decreases and now it would become equal to that of solid solvent at a lower temperature. The difference between the freezing point of the pure solvent and its solution is called depression in freezing point.
If T0f is the boiling point of the pure solvent and Tf is the boiling point of the solution then depression in freezing point is given as
∆Tf =T0f-Tf
Just like elevation in boiling point, depression in freezing point is also directly related to molality ‘m’.
∆Tf = 1000 x kf x m2 / M2 x m1
Where,
k f = molal depression constant
m2 = mass of solvent in g
M1 = mass of solvent in kg
M2 = molar mass of solute
Q10) Why do abnormalities in colligative properties occur?
A10) The colligative properties are dependent on the number of particles of the solute, in few cases when the solute dissociates or associates in the solution, abnormal results occur for molecular mass
As studied earlier the colligative properties of a solution depends solely on the number of particles of the solute and not on their nature. But sometimes while measuring colligative properties abnormal results are obtained due to the following reasons:
- When the solution is very concentrated, the particles present in the solution of the solute begin to interact with each other, therefore the solution should not be highly concentrated.
- In case of association of two or more molecules of solute associate together to form a bigger molecule, the number of effective molecules in the solution therefore decreases. As the colligative property is inversely proportional to molar mass, the molar mass of such particles when calculated on the basis of colligative property the value will be higher than the true molar mass of the solute.
- In case of dissociation of two or more particles of the solute in the solution, the number of effective solute particles increases. In such cases the value of the observed colligative property will be greater than that calculated on the basis of undissociated solute particles. The molar mass of the solute calculated from the measurement of colligative property will be lower than the true molar mass of the solute.
Van’t Hoff Factor
In order to account for extent of association or dissociation, Van’t Hoff introduced a factor 'i'.

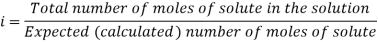
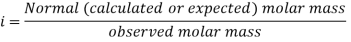
Degree of Association
Degree of association is defined as the fraction of the total number of molecules which associate to form a bigger molecule.
Degree of Dissociation
Degree of dissociation is defined as the fraction of the total number of particles that dissociate, i.e., break into simpler ions.
Q11) Define Raoults Law?
A11) Raoult’s law has been named after François-Marie Raoult, a French chemist who while conducting an experiment found out that when substances were mixed in a solution, the vapour pressure of the solution decreased simultaneously. Raoult’s law was established in the year 1887 and is also considered as the law of thermodynamics.
Raoult’s law states that a solvent’s partial vapour pressure in a solution (or mixture) is equal or identical to the vapour pressure of the pure solvent multiplied by its mole fraction in the solution.
Mathematically, Raoult’s law equation is written as;
Psolution = ΧsolventP0solvent
Where,
Psolution = vapour pressure of the solution
Χsolvent = mole fraction of the solvent
P0solvent = vapour pressure of the pure solvent
Q12) Define Henry’s law?
A12) Henry’s law refers to a gas law that provides an explanation about the dissolution of a gas in a liquid medium. This law states that the quantity of gas dissolved in a liquid is directly proportional to the partial pressure of that gas that is in equilibrium with the liquid. This can be given in an equation as below.
[A(aq)] = kH.PA(g)
Where, [A(aq)] is the concentration of the gas A that is dissolved in the solution,
kH is Henry’s law constant
PA(g) is the partial pressure of A(g)
Henry’s law constant is a proportionality constant and depends on the parameters like solvent, solute and temperature. Therefore, for a particular gas, the law varies at different temperatures Therefore, when calculating the solubility of a gas in water, one should obtain the value of Henry’s law constant at that particular temperature.
Q13) State the differences between Raoult’s law and Henry’s law and its applications?
A13) Henry’s Law: Henry’s law is a thermodynamic law that refers to the gas that is dissolved in a liquid medium.
Raoult’s Law: Raoult’s law is a thermodynamic law that gives an explanation about the relationship between the vapour pressure of a solution and the partial pressures of solutes in that solution.
Concept
Henry’s Law: Henry’s law states the quantity of gas dissolved in a liquid is directly proportional to the partial pressure of that particular gas and also is in equilibrium with that liquid.
Raoult’s Law: Raoult’s law refers to the vapour pressure of a solvent above a solution and is equal to the vapour pressure at that particular temperature, multiplied by the mole fraction of the solvent.
Proportionality Constant
Henry’s Law: The proportionality constant in Henry’s law is called Henry’s law constant.
Raoult’s Law: The Raoult’s law does not use a proportionality constant.
Conclusion
Henry’s law and Raoult’s law indicate the chemical behaviour of solutions that are in contact with their vapor pressures. The difference between Henry’s law and Raoult’s law is that Henry’s law explains the behavior of solutes of a solution whereas Raoult’s law explains the behavior of solvent in a solution.
Applications:
- Henry’s law in Carbonated soft drinks – When soft drinks bottle is opened some of the gas escapes giving a specific pop. This is due to the lower pressure above the liquid and carbon dioxide comes out as bubbles.
- Henry’s law in Respiration – The amount of oxygen that dissolves in the bloodstream is directly proportional to the partial pressure of oxygen in the alveoli air.
Raoults law: Two applications in chemistry:
Calculating the Vapor Pressure of a Solvent;
Calculating the Molecular Mass (Formula Weight) of a Solute
Q14) What are some of the important topics in “colligative properties”?
A14) Important topics in colligative properties of solutions include:
- Relative lowering of the vapour pressure of the solvent.
- Depression in freezing point of the solvent.
- Elevation in the boiling point of the solvent.
- The osmotic pressure of the solution.
- Van ’t Hoff Factor.
Q15) What are the important laws governing the colligative properties of solutions?
A15) The basic law governing the colligative properties of solutions is Raoult’s law. Raoult’s law explains the relationship between the vapour pressure of the solution, mole fraction and vapour pressure of the solvent. This can be given as:
p1= x1p1o
Where,
p1 = vapour pressure of solution
x1 = mole fraction of solvent
p1o= vapour pressure of the solvent
The law lays the fundamentals for the relative lowering of vapour pressure and further to the elevation in boiling point and depression in freezing point. It is also very important to know the limitations associated with the applicability of this law.
Q16) Define Dilute solutions?
A16) A dilute solution, refers to the solution in which the amount of the solute is less than the amount of the solvent. The behaviour of the dilute solutions is ideal as the changes in volume and heat occur, which is accompanied by mixing of solute and solvent and is negligible for all purposes. Dilute solutions obey Raoult’s law.
Dilution refers to the manner in which an additional solvent is added to a solution to decrease its concentration, however this keeps the amount of the solute constant, but increases the total amount of solution, thereby decreasing its final concentration.
Q17) What are the Limitations of Henry and Raoult’s law?
A17) When calculating the mole fraction of a solute, one should consider the number of moles of particles present in the solution instead of the number of moles of the compound added. For example, when an ionic compound is dissolved in water, each ion that is being separated in the solution should be considered as one particle (ex: NaCl gives Na+ and Cl- ions. Thus, the amount of particles present is twice the amount of NaCl added.)
Henry’s law can be used only if the molecules that are considered are in equilibrium. Moreover, this law does not work for high-pressure conditions. Moreover, if the dissolving gas shows a chemical reaction with the solvent, then this law cannot be used for that system.
Q18) When does the vapour pressure of the given liquid decrease?
A18) When a liquid is placed in a vessel that gets continually heated, the molecules of the liquid are seen to be moving at varying speeds in different directions. This happens due to the different kinetic energies possessed by the molecules of the liquid.
When the liquid is heated, the energy of the molecules rises; it becomes lighter and occupies the surface of the liquid. This process is known as ‘evaporation’. The molecules which can be seen on the liquid surface are called ‘vapor’.
The evaporation continues at a constant rate the temperature of the liquid is kept constant. When some molecules of the liquid in the vapour phase, strikes the walls of the containers or the surface of the liquid, it may get converted back to the liquid phase. This process is called condensation
Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with its condensed phases (solid or liquid) in a closed system at a given temperature. The equilibrium vapour pressure is known to serve as an indicator of the evaporation rate of a liquid. The propensity of particles to escape from the liquid (or a solid) is known to be related. A material that, at normal temperatures, has a high vapour pressure is generally referred to as a volatile material. It can be noted that the pressure exhibited above a liquid surface by the vapour is called vapour pressure
Q19) What is the relationship between Osmotic pressure and relative lowering of vapour pressure?
A19) Relation between osmotic pressure and lowering of vapour pressure
Van’t Hoff equation for dilute solutions is
π=VnRT.....(i)
In case of a dilute solution, The volume of solution can be taken as equal to that of the solvent. If N is the number of moles of the solvent of molecular weight M and density ρ, the volume V is given by
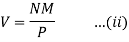
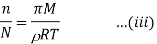
From Raoult’s law
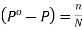 (iv)
(iv)
Or  [From (iii) and (iv)]
[From (iii) and (iv)]
The factor 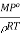 is constant at constant temperature
is constant at constant temperature

Or lowering of V.P∝ osmotic pressure
Q20) Is vapour pressure proportional to temperature?
A20) When a liquid’s vapour pressure is the same as the atmospheric pressure, the material is at temperature and pressure at the boiling/freezing point. Vapour stress depends on temperature. Raoult’s law states that the solution’s vapour pressure is directly proportional to the solvent’s mole fraction
As a result, there are lower boiling points of liquids with high vapour pressure. By heating a liquid and allowing more molecules to enter the atmosphere, vapour pressure may be increased. This begins at the point where the vapour pressure is equal to the boiling atmospheric pressure