Unit – 4
Bioinorganic Chemistry
Q1) Define Bioinorganic Chemistry.
A1)
There are several naturally occurring metal complexes that are involved in life processes in some way 1. Calcium is well-known for its role in the development of strong bones and teeth. Calcium, on the other hand, tends to relieve muscle cramps and triggers a variety of reactions in the human body. Magnesium is necessary for our cells to function properly. Magnesium is, in reality, the most abundant mineral within each cello, second only to potassium. Ca2+ and Mg2+, as well as Zn2+, Cu2+, Fe2+, and Mn2+, play a role in biological processes in the nucleus and are present in measurable quantities (10-2 to 10-4 mol) in the cells2. Mg2+ or Mn2+ concentration affects the active configuration of RNA. Enzymes in both plants and animals depend on the energy provided by magnesium to complete their tasks. Magnesium is believed to provide energy by stimulating the development of adenosine triphosphate (ATP), which provides energy to our body's billions of cells. Magnesium acts as a catalyst or activator in this reaction, as well as a variety of other physical processes.
Enzymatic behaviour, for example, in DNA-polymerase, as well as the effects of divalent ions like Mg2+, Mn2+, and Zn2+ on the rate of polymer synthesis, have all been studied.
Finally, we will discuss the progress of models for metal ion sites found in metal complexes of single metal ions with organic ligands that have oxygen, nitrogen, sulphur, or phosphorous donor atoms as donor atoms. Molecular models were first created to analyse the conformational properties of organic compounds. However, due to the presence of the metal ion, which introduces a number of geometries that the metals present in their compounds, and the lack of criteria for interactions with the metal ion, co ordination compounds are less studied. Most molecular mechanics programmes handle metal-ligand interactions by either defining a covalent bond between the metal ion and the ligand atom (the "bonded approach") or using electrostatic and Van der Waals forces (the "non-bonded approach")4. This analysis will not examine the methods; rather, it will describe the essence of the issues and the methods that have been devised to address them.
Q2) Explain The basis of chemical reactions of metalloenzymes.
A2)
In many cases, reaction environments are controlled by biopolymers such as proteins, and selective reactions are performed.
1. Coordinative activation (coordination form, electronic donation, steric effect)
2. Redox (metal oxidation state),
3. Information communication, and, in many cases, reaction environments are regulated by biopolymers such as proteins, and selective reactions are performed.
Other than metalloenzymes, metals can behave in a variety of ways.
1. Mg: Mg ATP
2. Pumping of Na/K ions,
3. Ca: Metals play important roles in hormone transfer, muscle contraction, nerve transmission, and blood coagulation, to name a few.
(a) Oxidation
Oxidation reactions in living systems are critical to life, and several studies have been conducted on them. The mechanisms of oxygen gas transportation by haemoglobin and mono-oxygen oxidation by iron porphyrin compounds known as P-450 have been extensively researched. The transportation of oxygen gas, which has been researched for many years, is listed below. The iron porphyrins haemoglobin and myoglobin, as well as the copper compound hemocyanin, are involved in the transfer of oxygen from the environment to living organisms' cells.
(b) Nitrogen fixation
All life depends on the reaction that transforms nitrogen in the air into ammonia. Nitrogen fixation, or the process of converting atmospheric nitrogen to ammonia, is carried out by Rhizobium in legume roots and bacteria in algae in an anaerobic environment. Before the invention of the Harber-Bosch process, all animals and plants, including humans, relied on biological nitrogen fixation as a source of nitrogen for protein and other nitrogen-containing compounds.
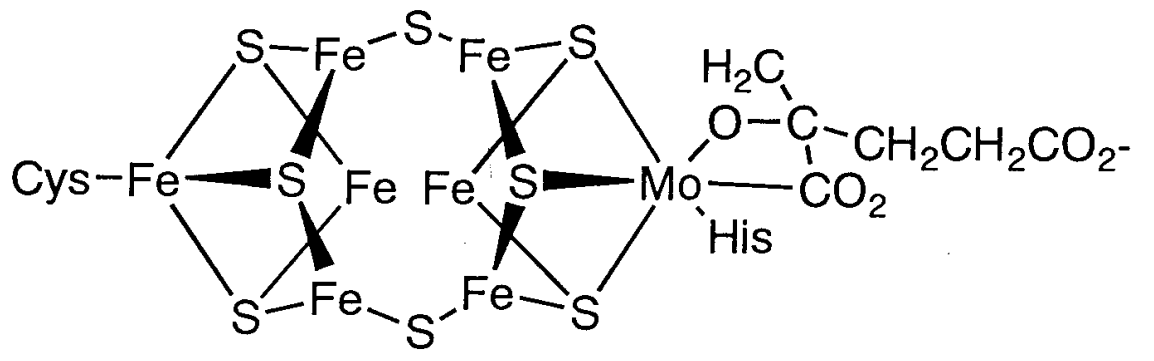
Figure: The Fe-Mo cofactor structure in nitrogenase.
Dinitrogen is thought to be triggered by coordination between the two clusters. The P cluster part, on the other hand, is made up of two Fe4S4 clusters. Both parts' functions and reaction mechanisms are still unknown.
(c) Photosynthesis
The reaction of carbon dioxide and water to produce glucose and dioxygen is a clever photochemical reaction in which chlorophyll (Figure 8.2.38.2.3), a magnesium porphyrin and manganese cluster complex, plays a key role. Photosystem I (PSI) and photosystem II (PSII) are photosystems that use light energy to eliminate carbon dioxide and oxidise water in chloroplasts.
Q3) Explain the Classification of elements according to their action in biological system.
A3)
Many scientific disciplines focus on the chemical elements found in organisms and their functions. Such investigations are supported by chemistry and biology. With the passage of time, more advanced disciplines have emerged.
Developed with emphasis on either organic or inorganic chemistry (Figure 1).
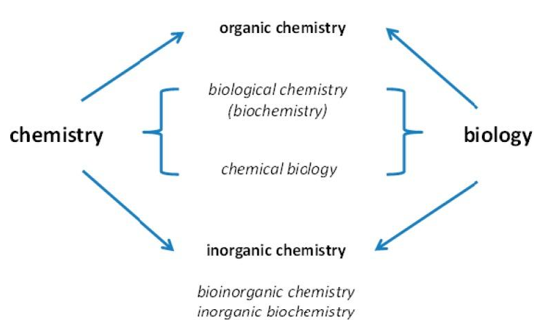
Investigations of the positions of chemical elements in species combine chemistry and biology.
When organic chemistry is concerned with life and inorganic chemistry is only associated with the inanimate environment, the names of the sub-disciplines may be misleading. Life is made up of both organic and inorganic compounds, and as I'll show later in this article, much more chemical elements are essential for life than those typically studied in organic chemistry. Many metal ions are among them, and they have played an important role in the evolution of life—life would not be possible without them. Metallomics is an applied biometal science that seeks to cover all facets of how metals work in biological systems.
There are three distinct trends that contributed to our current understanding, all of which are linked to a change in the types of questions answered and are still ongoing.
The Essential Elements
Given our vast understanding of genes and proteins, it's amazing that our understanding of the role of chemical elements in life is still minimal and open-ended. Bromine, which plays a role in collagen metabolism, was only recently added to the list of essential elements. As a result, we shouldn't presume that we know everything there is to know about the elements that are important for animals and humans, since certain chemical elements' biochemical functions are still unknown.
The Non-Essential Elements
Metal determination using instrumental analytical chemistry has progressed dramatically. With sub-ppt detection limits, inductively coupled plasma mass spectrometry (ICP-MS) can detect virtually all naturally occurring elements in biological samples. It's also possible to detect radioactive uranium. As a result, salmon eggs contained 74 of the 78 components. The abundance of chemical elements in humans has been summarized in a periodic table.
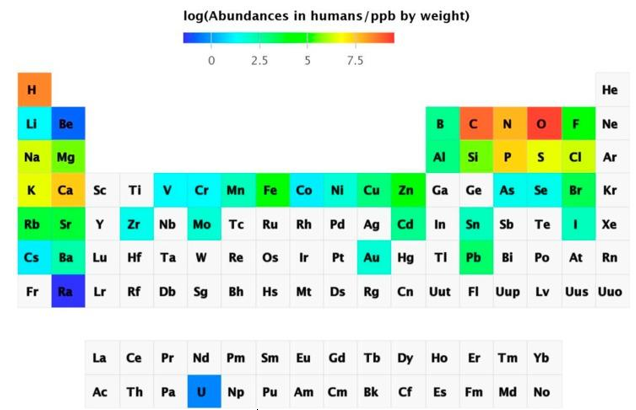
Q4) Explain Abundance of the chemical elements in humans.
A4)
Non-essential elements are found in far higher concentrations than essential elements, which are present in the lowest concentration. Sr is present at ppm levels, and Rb, Ba, Ni, As, Al, and Ti (not shown in Figure 3) are present at ppb levels, although there are also others that are thought to have primarily toxic effects, such as Cd, Hg (not shown in Figure 3), and Pb, which are variable and dependent on exposure. Some components accumulate over time, while others are tightly regulated. The existence of non-essential elements following a skewed distribution based on exposure has been proposed as a criterion for elements being essential, namely homeostatic regulation for essential elements leading to a normal distribution but the presence of non-essential elements leading to a skewed distribution. Although certain elements are bioactive and have beneficial health effects, many non-essential elements are also bioactive. They are not chemically inert and can function as catalysts, pharmacologically active agents, or toxic substances. As a result, it's important to take a wider view of metal ions in biology, one that extends beyond the emphasis on vital metal ions to include the many elements that are present but whose functions are unknown. We don't know how animals and humans cope with these seemingly non-essential components, whether their existence indicates a lack of selectivity in absorption processes, whether there are any unique detoxification mechanisms, or what the exposure limits and biological effects are.
Q5) Explain Na/K-pump.
A5)
The energy-intensive method of pumping molecules and ions through membranes "uphill" - against a concentration gradient - is known as active transport. A carrier protein is needed to transport these molecules against their concentration gradient. Carrier proteins can carry solutes along a concentration gradient (during passive transport), but some can also move solutes across a concentration gradient (from low to high concentration) with the addition of energy. Pumps are carrier proteins that are used to transfer materials toward their concentration gradient during active transport. ATP provides the energy for most active transport, just as it does for other forms of cellular activities. Transferring a phosphate group directly to a carrier protein is one way ATP drives active transport. This can cause the carrier protein to change shape, allowing the molecule or ion to cross the membrane to the other side. The sodium-potassium pump, shown in Figure below, is an example of an active transport system that exchanges sodium ions for potassium ions across the plasma membrane of animal cells.
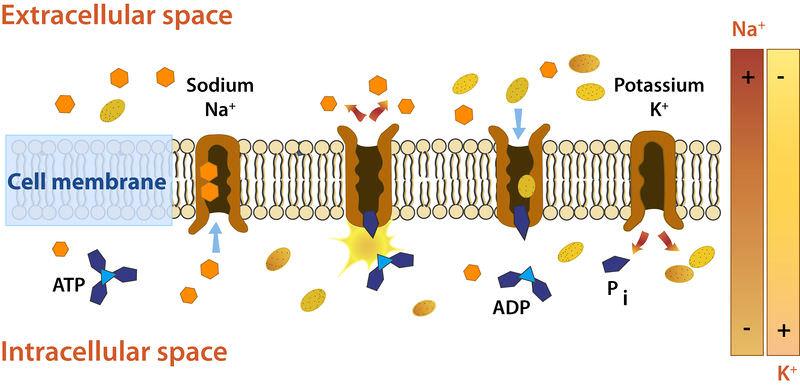
Sodium and potassium ions are moved by the sodium-potassium pump mechanism through broad concentration gradients. It pumps three sodium ions out of the cell and into the extracellular fluid, and it transfers two potassium ions into the cell where potassium levels are high.
Three sodium ions associate with the protein pump within the cell, as shown in Figure. The carrier protein then uses ATP to gain energy and change shape. It does so by expelling the three sodium ions from the cell. Two potassium ions from outside the cell bind to the protein pump at this stage. The potassium ions are then transported into the cell, where the cycle begins again. The sodium-potassium pump is located in almost every human cell's plasma membrane and is necessary for all cellular life. It controls cellular volume and helps to retain cell potential.
The Electrochemical Gradient
An electrical gradient form across the plasma membrane due to the active movement of ions across the membrane. Outside the cell, the number of positively charged ions exceeds the number of positively charged ions in the cytosol. As a result, the inside of the membrane has a comparatively negative charge, while the outside has a positive charge. The voltage across the membrane is caused by the charge difference. A separation of opposite charges, in this case through the membrane, causes voltage, which is electrical potential energy. Membrane potential is the voltage through a membrane. The conduction of electrical impulses along nerve cells is highly dependent on membrane potential.
Q6) What is Carbonic anhydrases?
A6)
Carbonic anhydrase is an enzyme that helps the conversion of carbon dioxide and water into carbonic acid, protons, and bicarbonate ions to occur quickly. This enzyme was first discovered in cow red blood cells in 1933. It has also been discovered in large quantities in all mammalian tissues, plants, algae, and bacteria. There are three types of this ancient enzyme (called alpha, beta and gamma carbonic anhydrase). Despite the fact that members of these various groups have little in common in terms of sequence or structure, they all serve the same role and need a zinc ion at the active site. Mammalian carbonic anhydrase is in the alpha class, plant enzymes are in the beta class, and the enzyme from methane-producing bacteria found in hot springs is in the gamma class. As a result, it's clear that these enzyme groups formed independently to produce a common active site. The alpha, beta, and gamma carbonic anhydrase enzymes are represented by PDB entries, which are shown from top to bottom. In these diagrams, the zinc ions in the active site are coloured blue. The alpha enzyme is a monomer, while the gamma enzyme is a trimeric complex. Despite the fact that this beta enzyme is a dimer, it has four zinc ions bound to it, suggesting four potential enzyme active sites. Other members of this class form tetramers, hexamers, or octamers, implying that dimer is most likely a building block.
Carbonic anhydrases in mammals exist in about ten slightly different ways, depending on the tissue or cellular compartment in which they are found. The sequence variations in these isozymes cause specific differences in their behaviour. As a result, isozymes present in certain muscle fibres have lower enzyme activity than salivary gland isozymes. While the majority of carbonic anhydrase isozymes are soluble and secreted, others are bound to epithelial cell membranes. Please visit the European Bioinformatics Institute's Protein of the Month feature for a more in-depth look at carbonic anhydrase from a genomic perspective.
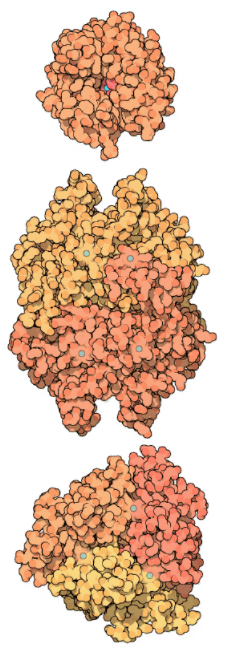
Alpha (top), beta (middle), and gamma (bottom) carbonic anhydrases.
Q7) Explain Carboxypeptidase and Substrate Binding.
A7)
Proteins are important molecules in organisms which serve a variety of roles throughout the human body, from supplying tensile strength to bones and tendons to storing and transporting essential substances like oxygen and iron in the body. As a result, proteins from foods must first be segregated into their constituent amino acids within the body's cells. The amino acids are then used to construct the proteins that our bodies need.
A hydrolysis reaction is used by the cell to break down a protein into its constituent amino acids. The protein binds to a water molecule, forming an amino acid and a new protein. The pancreas secretes the enzyme carboxypeptidase A, which is used to speed up the hydrolysis reaction. This enzyme is made up of a single 307-amino-acid chain, as shown in Figure 2. It takes on a compact, globular shape that contains helices and pleated sheet regions. This globular form has a pocket-like region where a substrate can be placed. The enzyme's active site is located here.
Since the active site changes significantly when the substrate binds, carboxypeptidase A is a clear example of the induced-fit principle. Figures 2 and 3 depict the carboxylase protein with and without a bound substrate. When the active site is complexed with a substrate, it changes shape. The active site of carboxypeptidase closes in around the protein substrate as it binds to it. If the terminal residue has an aromatic or bulky hydrocarbon side chain, hydrolysis of the peptide bond is more likely. At the active site, a zinc ion (Zn2+) is closely bound and aids in catalysis. For the enzyme to recognise the terminal amino acid in the peptide chain, three hydrogen bonding and electrostatic interactions are needed. Interactions with Zn2+ and the carboxypeptidase molecule stabilise the intermediate. Proton transfer and peptide bond cleavage are the final steps. This whole process necessitates a high level of mobility in the carboxypeptidase A protein.
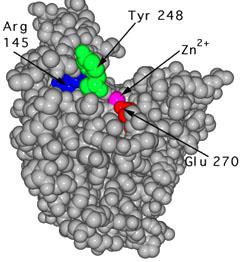 | 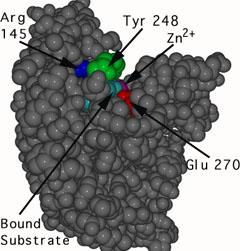 |
Figure: The unbound carboxypeptidase an enzyme is described by this molecular model. The cpk, or space-filled, representation of atoms is used to display the active site's estimated volume and form. | Figure: This is a cpk representation of carboxypeptidase A in which the active site has a substrate (turquoise) bound to it. The active site is in a conformation that has been caused. To show the shape shift, the same three amino acids (Arg 145, Tyr 248, and Glu 270) are numbered. |
Q8) Write short note on Trace Elements.
A8)
Trace elements (or trace metals) are minerals found in trace quantities in living tissues. Some are believed to be nutritionally important, others may be essential (though the evidence is only suggestive or incomplete), and the rest are deemed unnecessary. Trace elements are mainly used as catalysts in enzyme systems; however, certain metallic ions, such as iron and copper, are involved in energy metabolism's oxidation-reduction reactions. Iron, which is found in haemoglobin and myoglobin, is also essential for oxygen transport.
If ingested at high enough levels for long enough periods of time, all trace elements are toxic. For certain critical trace elements, the difference between toxic and ideal intakes to fulfil physiological needs is important, but for others, it is much smaller.
This chapter provides an overview of the roles of iron, zinc, fluoride, selenium, copper, chromium, iodine, manganese, and molybdenum in the aetiology and prevention of chronic diseases. Aluminum, cadmium, mercury, arsenic, and lead are also discussed; these elements have not been shown to be important for humans, but the committee considered them because they are commonly consumed as pollutants in food or water. Interactions among the various trace elements are briefly discussed as well.
Epidemiologic evidence on the connection between several trace elements and the occurrence of diseases like cancer, cardiovascular disease, and hypertension is lacking. Cadmium, chromium, and selenium have been the subjects of the majority of these tests. Furthermore, the majority of the literature does not pertain to dietary exposure, but rather to inhalation exposure in the workplace, for example. Animal feeding trials have also yielded inconclusive results. The committee discusses information gaps and recommends research directions.
Q9) Explain Cancer and their Types.
A9)
- Epidemiologic and Clinical Studies
Iron deficiency is a risk factor for the Plummer-Vinson (Paterson-Kelly) syndrome, which was once widespread in parts of Sweden but is now almost completely eradicated thanks to better nutritional status, especially in terms of dietary iron and vitamins (Larsson et al., 1975; Wynder et al., 1957). This disorder has been linked to an increased risk of cancers of the upper digestive tract, especially cancers of the oesophagus and stomach, indicating that underlying iron deficiency may be one of the factors contributing to the development of these cancers. Low dietary iron intake, on the other hand, has not been linked to cancers at these sites in epidemiologic studies (Schottenfeld and Fraumeni, 1982).
2. Animal Studies
Compared to 6 months in an iron-sufficient population, iron-deficient rats given 1,2-dimethylhydrazine (DMH) formed neoplastic liver lesions in 4 months (Vitale et al., 1978). The lack of iron tended to encourage carcinogenesis, according to the scientists.
In BALB/c mice with transplanted Merwin Plasma Cell-II tumours, the impact of iron deficiency on tumour growth and host survival was investigated (Benbassat et al., 1981). In weanling mice, an iron deficiency slowed body development and tumour growth, but not in adults. The cause of this disparity in response has yet to be identified. In iron-deficient female Wistar rats, mammary tumours induced by intragastric administration of dimethylbenz[a]anthracene (DMBA) and fibrosarcomas induced by subcutaneous injections of methylcholanthrene (MCA) were examined (Webster, 1981).
Animal research, in comparison to epidemiologic studies, show that iron can either stimulate or inhibit tumour growth depending on the circumstances. The effects of iron deficiency on tumour growth are controversial, and some iron compounds can act as cocarcinogens. The method of administration, the dosage, and the type of iron compound all seem to have an effect on the outcome.
3. Short-Term Tests
With and without metabolic activation by the S9 fraction, Brusick et al. (1976) discovered that Fe [II] as iron sulphate caused reverse mutations in Salmonella typhimurium strains TA1537 and TA1538. Another research looked at 45 metal salts to see whether they could cause morphological changes in Syrian hamster foetal cells in vivo. Positive transformation assays for iron were among the trace elements for which positive transformation assays were obtained (DiPaolo and Casto, 1979).
Q10) Explain Coronary Heart Disease and Their Types.
A10)
● Epidemiologic Studies
Iron deficiency is more common in women than in men, which has been suggested as an explanation for premenopausal women's lower coronary heart disease (CHD) incidence (Sullivan, 1986); however, no epidemiologic evidence supports this theory.
● Iron-Deficiency Anaemia
- Epidemiologic Studies
Iron deficiency anaemia occurs when the amount of iron in the body falls short of what is needed for the normal formation of haemoglobin, iron enzymes, and other iron compounds. It is the world's most common nutritional deficiency (Dallman et al., 1979) and the leading cause of anaemia in Western countries. However, in the United States, overall prevalence is poor. NHANES II showed that the highest prevalence (9.3%) occurred among children 1 to 2 years old; next came women age 15 to 19 (7.2%) and 20 to 44 (6.3%). Men aged 15 to 64 years old had a prevalence rate of less than 1%. (LSRO, 1984a).
2. Animal Studies
The hearts of rats with experimentally induced iron deficiency anaemia showed dramatic morphological changes. Cellular hypertrophy, as well as cellular degeneration and interstitial fibrosis, were prominent features of these shifts (Rossi and Carillo, 1983). Rossi and colleagues used reserpine to treat iron-deficient anaemic rats in other trials (Rossi and Carillo, 1982; Rossi et al., 1981). Cardiac hypertrophy was observed in the hearts of anaemic rats who were not given reserpine, as shown by increases in heart weight and muscle cell level. The hearts of animals given reserpine did not enlarge. The researchers hypothesised that noradrenaline can play a role in iron-deficiency anaemia-induced cardiac hypertrophy.
3. Zinc
Zinc plays an important role in nucleic acid metabolism, cell replication, tissue repair, and growth through its action in nucleic acid polymerases, which is found in over 200 enzymes. The potentially rate-limiting enzymes involved in DNA synthesis are among the zinc-dependent enzymes. Zinc also has a number of well-known and biologically significant interactions with hormones, and it plays a role in hormone synthesis, storage, and secretion. In the United States, serious, moderate, and minor zinc deficiencies have been identified (Hambidge et al., 1986).
Shellfish (especially oysters), beef, and other red meats are the best sources of zinc. Poultry, eggs, hard cheeses, milk, yoghurt, legumes, nuts, and whole-grain cereals are all healthy sources of this nutrient. Zinc absorption may be hampered by a variety of dietary factors, including other minerals, phytates, and dietary fibre (Hambidge et al., 1986). At the turn of the century, zinc has been used in a variety of foods. Until the middle of the 1930s, people got almost equivalent quantities of zinc from animal and plant foods, but since 1960, animal foods have accounted for roughly 70% of the food supply zinc. Zinc from animal sources tends to be more readily absorbed than zinc obtained from plants.
Q11) Explain Atherosclerotic Cardiovascular Diseases
A11)
● Epidemiologic and Clinical Studies
Klevay (1975) hypothesised that an excess of zinc relative to copper may underpin CHD based on knowledge of the relationships between zinc and copper and several risk factors for CHD, including elevated serum cholesterol and hypertension. For example, adding more than 10 times the RDA of zinc to the diets of 12 adult men for 5 weeks while maintaining normal copper levels resulted in a substantial decrease in high-density lipoprotein (HDL) cholesterol but no improvement in total cholesterol (Hooper et al., 1980). This theory, as well as the contradictory evidence supporting it, is explored in the copper section.
● Animal Studies
There were no animal studies on the connection between zinc and cardiovascular disease that the committee could find.
Q12) Explain Cancer in Brief.
A12)
● Cancer
- Epidemiologic and Clinical Studies
There have been few epidemiologic studies looking into the link between zinc exposure, especially dietary zinc, and cancer risk. Zinc levels in soil, food, and blood have all been linked to a variety of cancers in association studies (Schrauzer et al., 1977a, b; Stocks and Davies, 1964).
Blood and hair samples from a random sample of 58 men and 53 women who had undergone esophagoscopy with biopsy were tested for zinc, riboflavin, and vitamin A components in an attempt to classify etiologic factors for esophageal cancer in the very-high-risk region of Linxian in Hunan Province, China (Thurnham et al., 1982). Zinc levels in plasma and hair did not vary substantially between subjects with normal histology and those with lesions that were thought to be cancer precursors (e.g., esophagitis, dysplasia, acanthosis). Another analysis of a similar random sample from a low-risk region in Shandong Province showed no variations in zinc levels in blood and hair (Thurnham et al., 1985).
Just one case-control cancer study looked at dietary zinc. Kolonel et al. (1988) discovered that patients with prostate cancer aged 70 and up consumed more zinc (from supplements rather than food) prior to the onset of the disease than matched population controls. Occupational studies of workers who were exposed to zinc by inhalation (usually in the presence of other trace elements including copper, lead, arsenic, and chromium) have found no evidence that zinc is a cancer risk factor (Gerhardsson et al., 1986).
2. Animal Studies
Zinc has been shown to have both stimulating and inhibiting effects on tumour growth in animals, according to research. Several studies have shown that a zinc-deficient diet slows the development of transplanted tumours and increases survival time (Barr and Harris, 1973; Beach et al., 1981; DeWys and Pories, 1972; DeWys et al., 1970; Fenton et al., 1980; Mills et al., 1984; Minkel et al., 1979). These results indicate that tumour cells that are rapidly growing need zinc to develop. Severe zinc deficiency, with or without concomitant malignancies, is lethal in and of itself, so it is not recommended as a therapeutic modality.
In comparison to transplanted tumours, zinc deficiency tends to promote chemically induced carcinogenesis. Gabrial et al. (1982) found that in zinc-deficient rats, the frequency of esophageal tumours caused by nitroso methylbenzylamine was much higher than in control rats. In other research, high zinc intake suppresses the carcinogenesis of dimethylbenzylamine in Syrian hamsters (Poswillo and Cohen, 1971) and azo dyes in rats (Poswillo and Cohen, 1971). (Duncan and Dreosti, 1975).
Q13) Explain Toxic effect of metals.
A13)
● Two Classes of Toxic Metal Compounds
Excess quantities of an important metal may be just as harmful as insufficient amounts, as stated in the previous section. This condition may occur as a result of inadvertently ingesting the element or from metabolic disorders that render normal biochemical mechanisms that regulate uptake and distribution ineffective. One form of metal toxicity is represented by these possibilities. The entry of non-essential metals into the cell through food, skin absorption, or respiration is the other large category. Because of the public health threats posed by chemical and radio isotopic environmental contaminants, the toxicities associated with this latter class have gained a lot of attention recently.
● Copper Overload and Wilson's Disease
Wilson's disease is caused by a genetically inherited metabolic deficiency in which normal levels of copper are no longer tolerated. Liver disease, brain injury, and brown or green (Kayser-Fleischer) rings in the cornea of the eyes are the clinical symptoms. Wilson's disease patients have low levels of ceruloplasmin, a copper-storage protein; the gene and gene products responsible for the altered metabolism have yet to be identified. Chelation therapy, which uses K2Ca (EDTA) to replenish body calcium reserves depleted by EDTA coordination, 2,3-dimercaptopropan-1-ol (BAL, British Anti-Lewisite), or d-penicillamine to remove excess copper, eliminates symptoms. Copper is presumably removed as Cu(I) thiolate complexes by the sulfhydryl groups of the latter two compounds. Wilson's disease provides a great opportunity for modern methodologies to isolate and clone the gene responsible for the altered Cu metabolism, resulting in a rational treatment basis.
● Toxic Effects of Other Essential Metals
Most of the other metals mentioned in Table 9.1 are toxic when present in concentrations above their natural cellular levels. Vitamin D and parathyroid hormones regulate calcium levels in the body. Failure to control Ca2+ results in tissue calcification, stone formation, and cataract formation, a complex mechanism of which little is known (see Chapter 3). Chronic manganese poisoning, which may occur after inhaling metal-oxide particles, as in the case of Chilean miners, causes neurological symptoms that are close to Parkinson's disease. There has been evidence of neuronal injury. Fortunately, these metals are only used in a few instances.
● Plutonium: A Consequence of the Nuclear Age
Some of the chelating agents that were created to treat iron toxicity are now being used to treat plutonium poisoning. Salts and siderophores of diethylenetriaminepentaacetic acid (DTPA) are particularly effective. By tailoring the ligand to fully encapsulate the eight-coordinate Pu (IV) nucleus, some improvement over naturally occurring chelates has been made. While only a few people have been affected, ingestion of 239Pu, for example, as tiny particles of PuO2 at nuclear power plants, can be fatal. 239Pu releases high-energy ex particles, which cause cancers of the bone, liver, lung, and lymph nodes, to which transferrin transports it. Plutonium is one of the most toxic metals known, with a maximum tolerated dose of just 1.5 g. Now we'll look at some other, more traditional examples of industrial contaminants.
● Mercury Toxicity and Bacterial Resistance
Weathering of mercury's most common ore, HgS, red cinnabar, releases Hg (II) ions into the atmosphere. Organomercurials of the general formula RHgX, which are used in agriculture, have also ended up as radioactive waste in the forest. RHgX and HgX2 bind to sulfhydryl groups in proteins with a high affinity, causing neurological disease and kidney failure. Metallothionein is a common protein target for reducing mercury toxicity. In Minimata, Japan, 52 people died after consuming mercury-contaminated fish and crustaceans near a factory waste outlet in 1953, in a widely publicised situation. While mercury in its volatile, elemental form, Hg (0), is said to be nontoxic, its conversion to alkylmercury compounds by anaerobic microorganisms using a vitamin B-12 biosynthetic pathway poses a serious health risk.
● Cadmium and Lead Toxicity
Acute or chronic exposure to these heavy metals can cause gastrointestinal, neurological, and kidney toxicity, among other symptoms. The use of unleaded fuel and the elimination of lead-containing pigments from paint has reduced the amount of lead released into the atmosphere each year significantly. Alkaline batteries, pigments, and plating are all sources of cadmium. Chelation therapy with CaNa2(EDTA) (acute) or penicillamine (chronic) can be used to treat lead poisoning (chronic). While both Cd (II) and Pb (II) bind to thionine’s sulfhydryl groups, we know nothing about the molecular mechanisms through which these elements cause toxicity.
● Metals as Carcinogens
While most metal ions have been found to be carcinogenic, Ni, Cr, and, to a lesser degree, Cd are the three most potent cancer-causing metals. Many nickel-containing ores contain nickel subsulfide, Ni2S3, which has been extensively studied and shown to be carcinogenic in humans and other species. In vitro gene replication infidelity was increased, and bacterial DNA repair was altered, according to short-term bioassays that included mutagenesis.
Q9) What is Mineral Toxicity? Explain Its Effects.
A14)
Plants work in a similar manner. In reality, the entire universe operates on a delicate balance, and every living being in that world is bound by the law of balance. The precise proportions of the seven important micronutrients must be preserved. This leads to the cautious inference that too much leads to toxicity, while too little leads to deficiency, as previously mentioned.
Electron carriers, enzyme activation, providing osmoticum for turgor and development, maintaining charge balance, structural components, and more are all functions of mineral elements in plants.
● Effects of Mineral Toxicity
Plants with mineral nutrient deficiencies experience stunting, abnormal thickening, and darkening of roots, reduced growth, massive disruption of cell and cell walls, reduced branching, small changes in the pH of the cytosol, an inability of an enzyme to align correctly with a reactant, iron chlorosis, oxidative stress, chlorosis, destruction of chloroplasts, and iron chlorosis.
This begs the question of how this delicate balance or precise proportion is achieved. There is a simple solution to this; the concentration of the mineral ion in tissue reduces the dry weight of tissues by 10% and is considered harmful. Since each plant has different nutritional needs, weights, energy requirements, and other factors, these concentrations vary between plants.
Another factor to remember is that an excessive amount of one element prevents the absorption of another. The presence of manganese toxicity, for example, is shown by the existence of brown spots surrounded by chlorotic veins. Manganese competes with magnesium and iron for absorption in this region, as well as preventing calcium translocation in the plant's shoot apex. As a result, manganese deficiency in plants causes a deficiency in iron, copper, and calcium.
Carcinogenic agents have the ability to destroy the genome or disrupt the cells that participate in the metabolic process. Many radioactive compounds are thought to be carcinogenic, but their carcinogenic properties are caused by the radiations they emit. Carcinogenic agents include gamma rays and alpha particles. Tobacco smoke, some dioxins, and inhaled asbestos are examples of non-radioactive carcinogens. Tobacco smoke emits toxic gases such as carbon monoxide, which can cause cancer. Carcinogenic compounds are often assumed to be synthetic chemicals, but they may be natural or synthetic. Carcinogenic compounds do not have to be harmful right away; they are sneaky.
Q10) What is Drug Therapy in Brief?
A15)
The current arsenal of anti-malarial drugs includes a wide range of agents, but the most powerful seem to be those that influence processes related to haemoglobin (Hb) digestion in the parasite's food vacuole (Fig). This is a one-of-a-kind and crucial process for parasite growth, and the primary purpose of Hb digestion appears to be to provide ‘room' for parasites that are rapidly growing and dividing. Parasites may be able to use some of the catabolites as nutrients while neutralising the effects of the ones that are clearly harmful (Fig.).
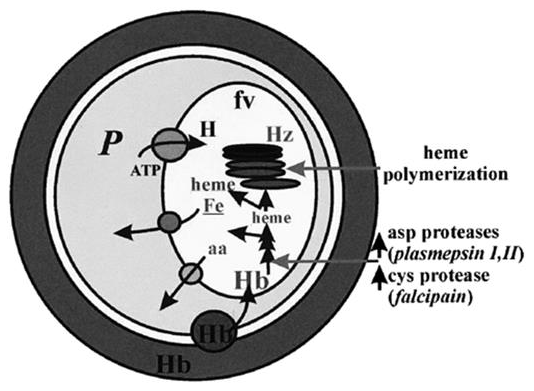
Intra-erythrocytic parasites' Hb digestion properties. Within the Hb-rich host cell, the parasite (P) is enclosed. Hb, along with other host components, is taken up by the parasite and transmitted to the parasite food vacuole through endocytosis (fv). The host-ingested Hb is degraded sequentially by the indicated Asp and Cys proteases in an acidic environment provided by an H+-ATPase in the fv. Part of the heme released is polymerized into hemozoin (Hz), while the rest can exit the fv and generate free Fe. Membrane transporters and/or simple diffusion systems appear to transport products of Hb digestion, such as amino acids (aa) and unpolymerized heme, into the parasite cytosol.
The uniqueness of that process is due to properties associated with the food vacuole, such as compartmentalised acidity and the expression of particular proteases for Hb degradation as well as mechanisms for neutralising the released toxic heme. Both heme polymerization into hemozoin and glutathione-mediated heme degradation have been proposed as mechanisms for the latter, with the first being linked to the food vacuole and the second to the parasite cytosol. The chemical or biochemical basis of those pathways, on the other hand, has yet to be determined. Nonetheless, it is clear that drugs that interfere (directly or indirectly) with either of the above processes of Hb digestion (e.g., CQ, anti-proteases, etc.) and/or heme neutralisation can jeopardise parasite survival (e.g., CQ, pro-oxidants). Furthermore, both heme, a product of Hb degradation, and iron, a putative product of GSH-mediated heme destabilisation, have been implicated in the catalysed development of toxic radicals by anti-oxidant drugs like artemisinin (Fig. Below). Due to the lack of mechanisms for iron absorption in mature red cells, rapidly growing parasites appear to rely on Hb degradation for a steady supply of the metal, making them susceptible to chemotherapeutic attack by iron chelators.
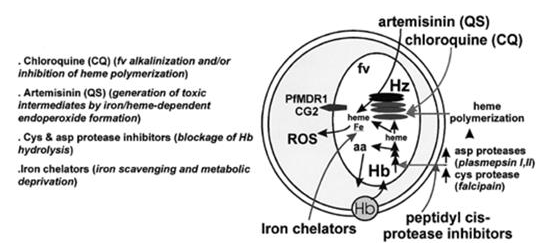
On parasite components, the site of action of the main anti-malarial drugs (AMDs). Long arrows (right) show the various drugs acting on different aspects of Hb degradation within the parasites, and their putative parasite targets are shown on the left side of the scheme. PfMDR1 and CG2 are putative drug transporters, and their presumed transport activities have been linked to parasite mdr products.
Q11) Write a short note on Iron and malaria.
A16)
Malaria parasites, like all living organisms, require iron for essential cell functions and must manage their cellular contents in a highly regulated manner. The relationship between the host's iron status and microbial infection has long been recognised. Microorganisms such as bacteria and fungi depend on extracellular iron (III) for growth and reproduction regardless of the environment. They get non-penetrating iron (III) by secreting siderophores, which are low-molecular-mass, water-soluble structures that scavenge extracellular iron (III) with high affinity, bind to specific surface receptors as iron complexes, and help iron internalisation through a multi-step mediated process. While no conclusive clinical success with bacterial infections has been identified, interference with those processes by bacterial receptor antagonists or iron deprivation at the host level has shown some differential effects on bacterial development. The general theory is that normal human tissue can tolerate temporary iron deprivation better than rapidly growing tumours, so iron chelation may be useful for cancer chemotherapy. Other applications have been proposed for treating conditions like cardiac ischemia reperfusion and adriamycin cardiotoxicity, which are both linked to iron-mediated cell damage. Above all, iron chelation therapy has proved to be highly effective and is still the most common treatment for iron overload.
Parasites have a unique case of iron metabolism during their asexual stage of development. They have no apparent means of mobilising bioavailable iron from the medium through the host, despite living in a sea of Hb and carrying within the remnants of its degradation products (Fig. 1). They differ from most mammalian cells in that they are exposed to body fluids and obtain metal from circulating iron carriers. The uniqueness of parasite iron handling is manifested in their resistance to drug-induced iron deprivation. What is the basis for iron (III) chelators' selective cytotoxicity, and what is the potential of iron chelation therapy in the treatment of malaria?
Q12) Explain Iron chelators as anti-malarial.
A17)
● Desferrioxamine (DFO)
Due to the malaria parasite's high replication rate and presumed high iron utilization, attempts have been made to cause selective "iron starvation" of parasites using the hydroxamate-based DFO, a natural siderophore and an iron (III) chelator that is currently used in clinical trials for iron overload diseases. The method seemed promising at first, as the agent inhibited P. Falciparum growth in vitro and suppressed malaria proliferation in mice, monkeys, and, more recently, humans. Inasmuch as DFO, like all other hydroxamates, had little effect on transferrin iron but demonstrated unique access into the inner compartments of infected cells, the source of iron affected by this highly specific iron chelator was hypothesised to be intracellular, probably intraparasitic. The drug's developmental stage of action was determined to be trophozoite/schizont, with the iron-dependent enzyme ribonucleotide reductase as the main putative goal (RNRase). DFO inhibits enzyme activity most likely by scavenging iron (III and II) from the iron reservoir with which the enzyme is in dynamic equilibrium. DFO may also function on parasites by destabilising the iron-containing hemozoin, resulting in the release of toxic heme-derived material, according to one theory. Due to reduced membrane permeation across biological membranes, DFO had a significant disadvantage in terms of speed of action in biological systems.
● New chelator design
The anti-malarial efficacy of the relatively safe DFO chelators could be improved by making hydroxamate-based drugs more permeant to infected cells. To achieve that goal, two methods were used, both of which were based on modular chemical approaches that allowed for systemic changes to improve drug performance. One is exemplified by a class I of synthetic hydrophobic siderophores, namely reversed siderophores (RSFs), which have an iron binding cavity biomimetic to that of ferrichrome, and the other, class II, is exemplified by N-terminal derivatives of DFO, which retain the parent compound's iron (III) binding capability.
Table:
Anti-malarial activity of various classes of iron chelators
Chelator | Class | IC50 in vitro (µM) | Rodents |
DFO | I | 10–30 | Sc, ip, iv |
X-DFO | I | 3.0–30 | NA |
RSF-tripodal | II | 0.3–20 | Sc |
HPO | III | 10–50 | Iv |
XIH | IV | 0.2–30 | Sc, ip |
DiOH-coumarin | V | 0.8–20 | Sc, ip |
The growth test was based on nucleic acid synthesis, which occurs most frequently at the trophozoite-schizont stage, and the IC50 values of the indicated classes of drugs were for a 24–48-h drug exposure in a mixed in vitro culture containing primarily trophozoites (>70 percent), and the IC50 values of the indicated classes of drugs were for a 24–48-h drug exposure in a mixed in vitro culture containing primarily trophozo RSF-tripodal refers to hydroxamate-based RSFs, HPO to hydroxypyridinons, XIH to aryl-aldehyde-isonicotinoyl hydrazone adducts, and diOH-coumarin to daphnetin, a bioflavonoid chelating agent. The indicated drugs were administered intravenously (iv), intraperitoneally (ip), and subcutaneously (sc) to rodent models of malaria (NA=not applied) and were successful in reducing parasite burden or parasitemia.
The effect of drug lipophilicity on the structure-activity relationships of three groups of iron chelators as anti-malarial. The drugs were tested in vitro on P. Falciparum development, and the IC50 values were calculated after a 48-hour exposure to the drugs, starting at the mid-ring level. The abscissa denotes the drugs' related chemical properties, which are calculated by the product of relative iron-drug binding affinity and drug partition coefficient (modified from with additional unpublished data on HPOs obtained by Haupt, Hider and Cabantchik). DFO derivatives (MA=methylanthranilic and NBD=nitrobenzyldiazole), different hydroxypyridinons (HPO), and substituted RSFs with different amino acids and connecting arms (m1 and m2) and/or ester groups were the three families of chelators studied (OEt, for ethyl).
● Specificity of action
To the extent that the above iron (III) chelators' efficacy on malaria parasites is based on their ability to cause a deficiency in bioavailable cell iron, this property is likely to occur to some degree in other cells of the host organism. As a result, the rationale for treating malaria with drug-mediated iron deprivation must presume that the host and parasite have different levels of drug tolerance. In practise, chelators must also meet the requirement of parasite cytotoxicity specificity versus host organism in order to be effective curative agents. Most mammalian cells tested for drug-induced growth arrest were found to be in this state. Despite the fact that almost all of the chelators used stopped the mammalian cells from growing, they resumed normal growth after the medication was removed and their serum iron pools were replenished (i.e., cytostatic action).
Q13) Write a short note on Haemoglobin.
A18)
Haemoglobin is a globular protein found in red blood cells (RBCs) that transfers oxygen across the body via the bloodstream. The heme prosthetic group is attached to each subunit of this tetrameric protein. It is a respiratory pigment that aids in the transport of oxygen from the lungs to various parts of the body as oxyhaemoglobin. Carbon dioxide is also transferred back into haemoglobin in the form of carbaminohaemoglobin.
Myoglobin in muscles, haemocyanin in arthropods and mollusks, leghaemoglobin in legumes, and other oxygen-binding proteins are examples.
Haemoglobin is a protein that carries oxygen in the body. HBA1, HBA2, and HBB genes code for a present in humans. The amino acid sequence in Hb polypeptide chains varies between organisms.
Hb heme is made in the mitochondria and cytoplasm of immature RBCs. Ribosomes produce the globin protein in the cytoplasm. The residual rRNA in mature mammalian RBCs continues to synthesise Hb until the reticulocytes reach the vasculature, even after the nucleus has been lost.
The level of haemoglobin in the blood is measured in g/dL. The amount in a healthy person varies from 12 to 20 g/dL. In general, males have a higher Hb level than females. Males have a normal level of 13.5 to 17.5 g/dL, while females have a normal level of 12 to 15.5 g/dL.
Let's take a closer look at haemoglobin's structure and work.
Structure of Haemoglobin
In 1959, Max Perutz discovered the molecular structure of haemoglobin. The protein haemoglobin is tetrameric. In adults, the main form of haemoglobin is made up of two polypeptide chains, each with two subunits. Each polypeptide chain has a heme prosthetic group attached to it.
Subunit – It is made up of a 141-amino-acid-residue alpha polypeptide chain.
Subunit – It is made up of a 146-amino-acid-residue beta polypeptide chain.
Heme group – Each polypeptide chain has an iron-containing prosthetic group attached to it. The porphyrin ring contains iron in the middle.
There is a close interaction between and subunits in the quaternary system. Haemoglobin partially dissociates after a mild urea injection, but dimers remain intact. Hydrophobic interactions, hydrogen bonding, and a few ion pairs or salt bridges bind the subunits together.
There are two alpha and two gamma chains in children, which are gradually replaced by beta chains.
There are two types of haemoglobin conformations: R state and T state. Deoxyhaemoglobin is mainly present in T state, while oxygen has a stronger affinity for R state.
Function of Haemoglobin
Hb's primary role is to carry and transport oxygen to different tissues. Oxygen binding to Hb is a cooperative process. The transition between the low oxygen affinity T state (Tense) and the high oxygen affinity R state (Ren) is responsible for the binding and release of oxygen from Hb in the lungs and tissues, respectively (Relaxed).
Q14) Explain the Transport of oxygen in Hemoglobin.
A19)
PH, 2,3 BPG, and BPG influence the affinity of oxygen for Hb (2,3-Bisphosphoglyceric acid). T-state is favoured in tissues with low pH, high BPG, and CO2, and oxygen is released, while R-state is favoured in the alveoli with high pH, low CO2, and BPG concentrations, resulting in oxygen binding to Hb.
The partial pressure of oxygen also influences oxygen binding. When pO2 is high in the lungs, oxygen binds with Hb, and when pO2 is low in the tissues, oxygen is released.
Each 100 millilitres of oxygenated blood carries 5 millilitres of oxygen to the tissues.
As the first oxygen molecule binds to the heme unit of one deoxyhaemoglobin subunit (T-state), conformational changes occur, resulting in an improvement in affinity, and the second molecule binds more quickly. When the fourth molecule is already in the R state, it binds to it. The sigmoid curve of oxygen binding to Hb can be seen.
Allosteric binding is a form of binding in which binding at one site affects the affinities of the other binding sites.
The pulse oximeter is a device that tests the amount of oxygen in the blood. It's used to figure out whether you're suffering from hypoxia. It is based on the fact that the absorption spectra of oxyhaemoglobin and deoxyhaemoglobin are different. This is a crucial method that doctors use to monitor COVID-19 patients' oxygen saturation levels, as well as those that are at risk.
Q15) Explain the Diseases related to Hemoglobin.
A20)
Haemoglobin deficiency can be caused by a variety of factors. The blood's oxygen-carrying ability is reduced as a result of haemoglobin deficiency. It may be caused by a lack of nutrients, cancer, kidney failure, or genetic defects.
Haemoglobin levels that are higher than average is linked to a variety of heart and pulmonary disorders.
Sickle cell anaemia is caused by a haemoglobin gene mutation. The globin chain has a single nucleotide or point mutation. Glutamic acid is replaced by valine at the 6th position after ‘GAG' is converted into ‘GTG.'
Thalassemia is caused by a decrease in haemoglobin production. -thalassemia and -thalassemia are the two forms of thalassemia. It is often caused by faulty genes, and the magnitude is determined by the number of genes that are absent or defective.
The haemoglobin level is a popular diagnostic tool. The HbA1c level, or glycosylated Hb or Hb linked to sugar, is a marker for a diabetic patient's average glucose level in the blood.
To summarise, haemoglobin is a necessary pigment for the transport of oxygen and the performance of normal bodily functions.