Unit - 1
Nitrogen Containing Functional Groups
Q1) What are the Preparation and important reactions of nitro and compounds, nitriles?
A1)
Preparation of Amines
Amines are prepared by the following methods:
o Nitro compound reduction
o Alkyl halide ammonolysis
o Nitrile reduction
o Amino acid reduction
Phthalimide synthesis by Gabriel
o Degradation of Hoffmann bromamide
- Reduction of nitro compounds
- By moving hydrogen gas through finely divided nickel, palladium, or platinum, nitro compounds are reduced to amines, as well as by reduction with metals in an acidic medium.
R- NO2 + 3H2 à R-NH2 + 2H2O {In the presence of Ni, Pt or Pd}
Ar- NO + 3H2à Ar- NH2 + 2H2O
- Nitro alkanes can also be reduced to the corresponding alkanamines in the same way.
- Reduction with iron scrap and hydrochloric acid is favoured because the FeCl2 produced during the reaction is hydrolyzed, releasing hydrochloric acid, requiring only a small amount of hydrochloric acid to start the reaction.
- Nitro compounds can also be reduced with active metals like Fe, Sn, Zn, and others using concentrated HCl.
R- NO2 + 3H2 à R- NH2 + 2H2O {In the presence of Sn/HCl}
Ar- NO2 + 3H2 à Ar- NH2 + 2H2O
II. Ammonolysis of alkyl halides
- In alkyl or benzyl halides, the carbon-halogen bond can be easily broken.
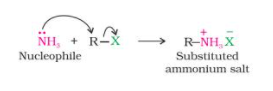
- As a result, when an alkyl or benzyl halide reacts with an ethanolic solution of ammonia, the halogen atom is substituted by an amino (–NH2) group in a nucleophilic substitution reaction.
- Ammonolysis refers to the cleavage of the C–X bond by an ammonia molecule.
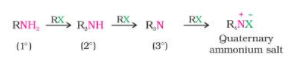
- At 373 K, the reaction is carried out in a sealed tube. The resulting primary amine acts as a nucleophile, allowing it to react with alkyl halides to produce secondary and tertiary amines, as well as the quaternary ammonium salt.
- Treatment with a solid base yields the free amine from the ammonium salt:
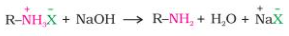
- Cons: When ammonia is consumed in excess, primary amine is formed as the main product.
- As alkyl halide is used in excess, the main product is quaternary ammonium salt.
- Note: This method is not appropriate for the preparation of aryl amines because aryl amines are less reactive towards nucleophilic substitution reactions than alkyl halides.
III. Reduction of nitriles
- Primary amines are formed when nitriles are reduced with lithium aluminium hydride (LiAlH4) or hydrogenated catalytically.
- This reaction is used to ascent the amine sequence, that is, to make amines with one more carbon atom than the starting amine.
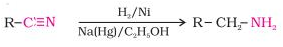
Q2) Explain the methods of preparation of Cyanides.
A2)
Methods of preparation of Cyanides
1. Dehydration of Amides:
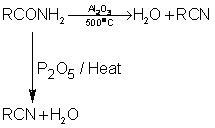
High molecular weight acid amides are dehydrated to the corresponding cyanide by heat alone.
CH3(CH3)6 OCNH2  CH3(CH2)6 CN
CH3(CH2)6 CN
2. From RX:
RX + KCN ——> RCN + KX
This method is satisfactory only if R is 1o or 2o group. If it is 3o group, then it is converted into alkene.
CH3CH2Cl + KCN → CH3CH2CN + KCl
3. By Grignard’s reagent and Cyanogen chloride reaction:
RMgCl + CICIN → RCN + MgCl2
This is best method for preparing 3o alkyl cyanides.
(CH3)3CMgCl + CICN → (CH3)3 CCN + MgCl2
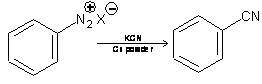
4. From Diazonium salt
Q3) Explain Amines.
A3)
Amines are aliphatic and aromatic ammonia compounds. Amines (K b = 10 4) and ammonia (K b = 10 6) are also weak bases. The unshared electron pair on the nitrogen atom causes this basicity.
Classification and nomenclature of amines:
The number of carbon-containing groups attached to the nitrogen atom determines whether amines are listed as principal, secondary, or tertiary. Primary amine compounds have just one group attached to the nitrogen atom, while secondary and tertiary amine compounds have two or three groups attached to the nitrogen atom, respectively.
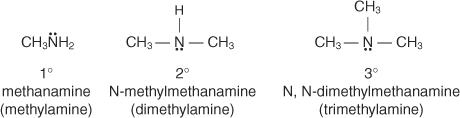
In the common system, you name amines by naming the group or groups attached to the nitrogen atom and adding the word amine.
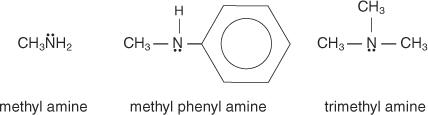
To call amines in the IUPAC System, use the following rules:
1. Identify the longest continuous carbon atom chain. The alkane with the same number of carbons is the parent’s name.
2. Change the alkane's e to "amine."
3. Identify and label any substituents, bearing in mind that the chain is numbered in the opposite direction of the amine group. A capital N is used to designate substitutes that are bound to the nitrogen atom rather than the carbon of the chain.
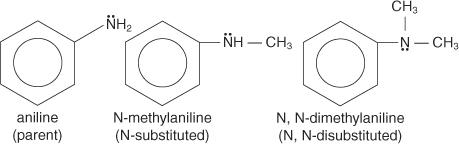
Aromatic amines are divided into several families, each of which acts as a parent molecule. An amino group (—NH 2) attached to benzene, for example, produces aniline as the parent compound.
Basicity of amines:
Amines are classified as basic because they have two unshared electrons that they can share with other atoms. The electron density around the nitrogen atom is produced by these unshared electrons. The more electron density there is in a molecule, the more fundamental it is. The basicity of amines is increased by groups that donate or supply electrons, while groups that decrease the electron density around the nitrogen decrease the basicity of the molecule. The following is the order of base strength for alkyl halides in the gas phase:
(CH 3) 3 N > (CH 3) 2NH > CH 3NH 2 > NH 3
Most least
Basic basic
|
However, in aqueous solutions, the order of basicity changes.
(CH 3) 2 NH > CH 3NH 2 > (CH 3) 3N > NH 3
Most least
Basic basic
|
Solvation results cause variations in the basicity order in the gas phase and aqueous solutions. Amines occur as ammonium ions in water solutions.
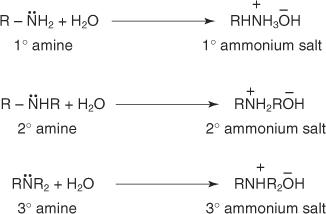
Main and secondary amines' ammonium salts undergo solvation effects (due to hydrogen bonding) in water to a much greater extent than tertiary amines' ammonium salts. The inductive effect of alkyl groups does not increase the electron density on the amine nitrogen as much as these solvation effects do.
Because of resonance, arylamines are weaker bases than cyclohexylamines. Figure 1 shows the resonance structures of aniline, a common arylamine.
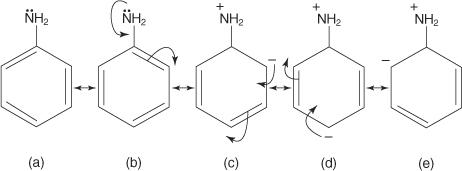
Delocalization of the unshared electron pair occurs in the ring, as seen in structures b through e in Figure, making these electrons less available for reaction. The molecule becomes less fundamental as a result of this electron delocalization.
Q4) What is Structure of Amines?
A4)
● Alkyl Amines
Tetrahedral nitrogen centres make up alkylamines. The bond angle between C-N-C and C-N-H is 109 degrees in this case. As compared to the C-C set, the distance between C-N is shorter. The amines may also be chiral, meaning that the nitrogen centre contains four replacements that form lone pairs.
● Aromatic Amines
Aromatic amines (also known as "anilines") have a nearly planar structure of nitrogen. This is due to the aryl substituent mixing with the lone pair. The C-N spectrum is a lot smaller. The difference between C and N in aniline is the same as the distance between C and C.
Examples:
H2N –(CH2)6 – NH2 (Hexane -1,6-diamine or 1,6-hexane diamine)
Q5) Explain the Preparation and properties of dinitrogen.
A5) Preparation of dinitrogen:
Commercially, nitrogen is obtained by liquefaction and fractional distillation of air. There are two key phases in this procedure:
Step 1: High pressure between 100 and 200 atmospheres is used to reduce air to liquid air. The compressed air is then expanded by passing it through a fine jet. This process is replicated several times, resulting in liquid air formation.
Step 2: Fractional distillation is performed on the resulting liquid. Dinitrogen has a lower boiling point than liquid oxygen, so it separates from it, leaving liquid oxygen behind. The impure liquid serves as a source of nitrogen.
In laboratory, dinitrogen is obtained by reacting aqueous solution of ammonium chloride with sodium nitrite.
N2(g)+ 2H2O(l) + NaCl = NH4Cl(aq) + NaNO2(aq) (aq)
Impurities such as NO and HNO3 are present in the material, which can be eliminated by thermal decomposition of ammonium dichromate. Passing the gaseous mixture by sulphuric acid containing potassium dichromate is another way to eliminate impurities.
N2+ 4H2O+ Cr2O3 (NH4)2Cr2O7
Pure nitrogen is formed during the decomposition of sodium or barium azide in the presence of high temperature.
Physical properties of Dinitrogen:
In nature, nitrogen is colourless, odourless, and diamagnetic. It's a non-toxic gas that's only slightly soluble in water.
Nitrogen condenses to form a colourless vapour, which then solidifies to form a snow-like mass.
Chemical properties of Dinitrogen:
Because of the N = N bond, dinitrogen has a high bond enthalpy. It is inert at room temperature as a result of this. However, as the temperature rises, the reactivity rises with it. Nitrogen molecules react with metals to form ionic nitrides and non-metals to form covalent nitrides at high temperatures.
2Li3N 6Li +N2heat 6Li +N2heat 6Li +N2heat 6Li +N2heat 6Li +
In Haber's Process, it reacts with hydrogen to form ammonia at 773 K.
773k 2NH3(g) + 3H2(g) N2(g) + 3H2(g) N2(g) + 3H2(g) N2(g) + 3H2(g) N
When a nitrogen molecule interacts with an oxygen molecule at a temperature of 2000 K, nitric oxide is formed.
2NO = N2(g) + O2(g) (g)
Dinitrogen's Applications:
It is primarily used in the industrial production of compounds like ammonia and calcium cyanamide.
It is used to create an inert environment in manufacturing industries such as iron and steel.
Liquid nitrogen is used as a preservative and a refrigerant in the food industry.
Q6) Explain Preparation of Dioxygen.
A6)
The catalytic decomposition of solid potassium chlorate is the most popular and practical method for producing dioxygen in the laboratory. The catalyst in this reaction is manganese dioxide.
2KClO3 = 2KCl +3O2 = 2KCl +3O2 = 2KCl +3O2 = 2KCl +3O2 = 2
MnO2 (Manganese Oxide)
The thermal decomposition of metal oxides from the lower part of the electrochemical sequence is another laboratory process. Dioxygen is generated by the thermal decomposition of silver oxide or mercuric oxide, for example.
2Ag2O → 4Ag + O2
Silver oxide Δ Silver Dioxygen
2HgO → 2Hg + O2
Mercuric oxide Δ Mercury Dioxygen
Dioxygen may also be obtained in the laboratory by heating the higher oxides of some metals like lead, barium, and manganese.
2PbO2 → 2PbO + O2
Lead (IV) oxide Δ Lead (II) oxide Dioxygen
2BaO2 → 2BaO + O2
Barium peroxide Δ Barium oxide Dioxygen
2MnO2 + 2H2SO4 → 2MnSO4 + 2H2O + O2
Manganese Sulphuric Δ manganese Water Dioxygen
(IV) oxide acid (II) sulphate
Salts that are rich in oxygen, such as permanganates and nitrates, when decomposed thermally also yields dioxygen.
● 2KNO3 → 2KNO2 + O2
Potassium Δ Potassium Dioxygen
Nitrate nitrite
● 2KMnO4 → K2MnO4 + MnO2 + O2
Potassium Δ Potassium manganese Dioxygen
Permanganate manganate (IV) oxide
● 2NaNO3 → 2NaNO2 + O2
Sodium Δ Sodium Dioxygen
Nitrate nitrite
Q7) Explain Gabreil Phthalimide synthesis in details.
A7)
There are three stages in the Gabriel Phthalimide Synthesis Mechanism. The Synthesis is named after German scientist Siegmund Gabriel and is used to make primary amines from primary alkyl halides.
The reaction has been generalised for use in the alkylation and deprotection of sulfonamides and imides to produce amines. Since ammonia alkylation is inefficient, it is replaced with phthalimide anion in the Gabriel synthesis.
Synthesis Details
The Gabriel synthesis has the greatest benefit of avoiding over alkylation. The reaction of potassium hydroxide with phthalimide also produces a strong nucleophile in the form of an imide ion. The imide ion performs a nucleophilic substitution reaction on the alkyl halide, yielding N-alkyl phthalimide as an intermediate. This phthalimide is hydrolyzed or hydrazinolyzed to produce a primary alkyl amine. Aryl amines, on the other hand, cannot be made using Gabriel synthesis because aryl halides do not undergo simple nucleophilic substitution.
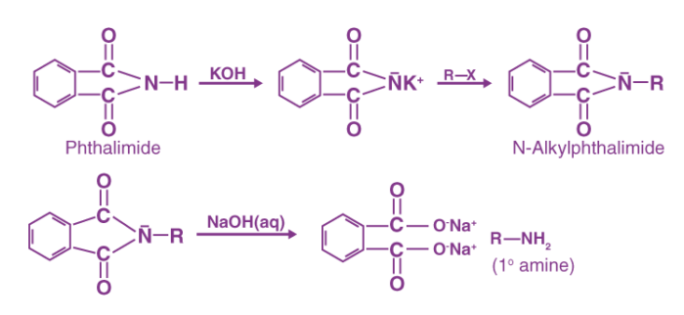
Gabriel Phthalimide Synthesis Mechanism
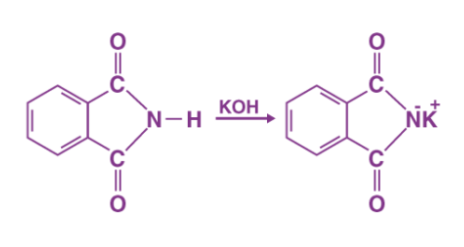
Step 1: An acid-base reaction occurs when potassium hydroxide is added to the phthalimide. The imide is deprotonated by the hydroxide ion. The resulting proton is more acidic than any simple amine (resonance stabilisation provided by the two adjacent carbonyl-like groups), resulting in a heavy nucleophile – the imide ion.
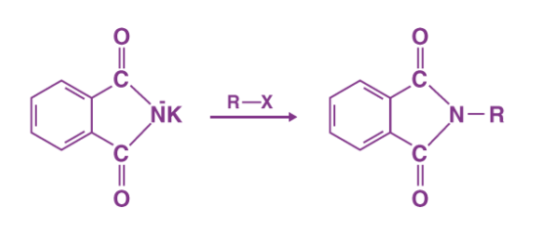
Step 2 The alkyl halide's electrophilic carbon is attacked by the nucleophilic imide ion. The nitrogen atom then bonds with the carbon itself, replacing the halogen (Fluorine, Chlorine, Bromine, or Iodine) in the alkyl halide. An N-Alkyl Phthalimide is formed as a result of this reaction.
3rd phase
The mechanism is very similar to base-catalyzed hydrolysis of esters, but instead of oxygen, nitrogen is added to the R group. The hydroxide ion cleaves the N-Alkyl phthalimide by attacking the carbon atom bound to the nitrogen atom. The oxygen atom is also attached to the cation in the base. As the oxygen atom replaces the nitrogen atom in the phthalimide, the hydrogens expelled from the hydroxide ion bind with the nitrogen atom attached to the R group. Below is an illustration of the third step in the Gabriel phthalimide synthesis mechanism.
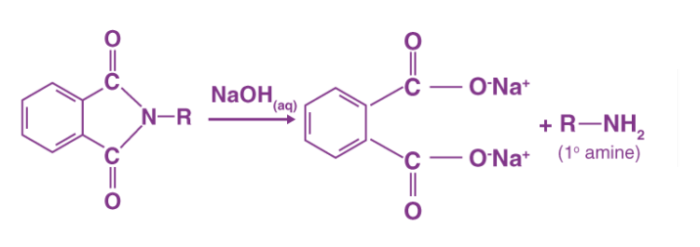
Finally, the Gabriel method can be used to synthesise primary amines from phthalimides. Instead of using an aqueous base, acidic hydrolysis or hydrazinolysis may be used to follow the mechanism (as was shown in the mechanism above). With secondary alkyl halides, the Gabriel approach typically fails. Another downside of this synthesis is that it produces a low yield by using acidic/basic hydrolysis, while using hydrazine will make the synthesis conditions relatively harsh.
Q8) What is Carbylamine reaction?
A8)
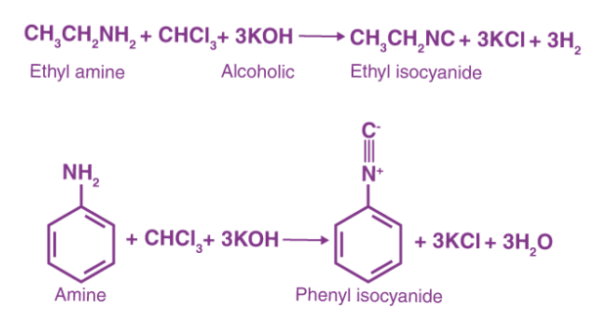
The addition of amine to the intermediate formed by the dehydrohalogenation of chloroform is part of the carbylamine reaction mechanism. Dichlorocarbene is the name of this intermediate. Hofmann isocyanide synthesis is another name for the carbylamine reaction. Isocyanides are made by combining a primary amine, chloroform, and a base. This conversion relies heavily on the dichlorocarbene intermediate. Isocyanides can't be made from secondary or tertiary amines using the carbylamine reaction. The carbylamine reaction can be written as – in general.
R-NH2 + CHCl3 + 3KOH —→ RNC (Carbylamine) + 3KCl + 3H2O
Here are a few examples of the carbylamine reaction.
Hofmann’s Isocyanide Test
The carbylamine reaction can be used as a chemical test for the existence of primary amines since it is only selective for primary amines. The carbylamine reaction is also known as Hofmann's isocyanide test when used as a test. The test substance is heated with chloroform and alcoholic potassium hydroxide in this procedure. In the presence of primary amine, isocyanide (carbylamine) can form, which is readily identifiable by its foul odour. Since secondary and tertiary amines do not undergo the carbylamine reaction, the Hofmann isocyanide test does not produce a foul odour.
Carbylamine Reaction Mechanism
To make dichlorocarbene intermediate, the first step is to dehydrohalogenate chloroform (remove hydrogen halide from a given substrate). The dichlorocarbene intermediate is a highly reactive intermediate. The electrophilic dichlorocarbene targets the primary amine's nucleophilic nitrogen. Isonitrile is formed when hydrochloric acid is removed from the system. The mechanism of the carbylamine reaction is depicted in the diagram below.
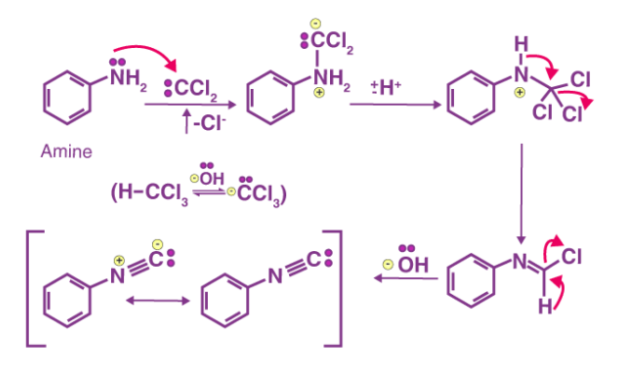
Finally, using chloroform and a base, the carbylamine reaction can be used to make isocyanides from primary amines. The carbylamine reaction can also be used to determine if a substrate contains a primary amine.
Reaction of Mannich
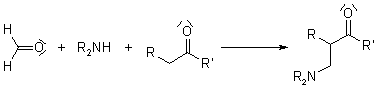
Aminomethylated products are generated by combining a nonenolizable aldehyde, a primary or secondary amine, and an enolizable carbonyl compound in a multi-component condensation. The acceptor in the reaction is the aldehyde's iminium derivative.
Many biosynthetic pathways, especially for alkaloids, have been proposed to include the Mannich Reaction.
Q9) Write a short note on Hoffmann s exhaustive methylation.
A9)
The Hofmann Elimination of Alkylammonium Salts: Examples and Mechanism
1. Quick Review: Zaitsev’s Rule
Zaitsev's law governs traditional elimination reactions involving the E2 process. The more substituted alkene would be the main product (that is, the alkene with the most carbons directly attached to the alkene).
The “trisubstituted” alkene is preferred over the “mono-substituted” alkene in the first example below.
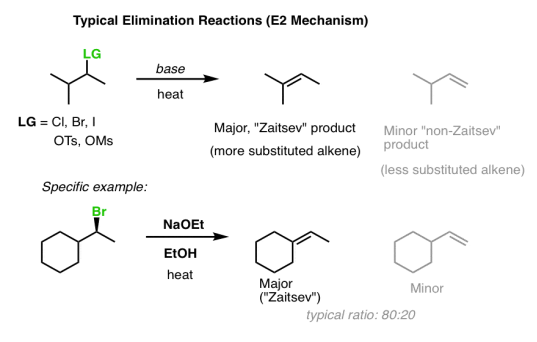
What is the reason for this? Alkenes' thermodynamic stability rises in order of monosubstituted, disubstituted, trisubstituted, and tetrasubstituted.
The energy differences are minor – around 2 kcal/mol – but they are sufficient to produce an 80:20 product ratio! [How did we come to this conclusion? It's calculated by substituting 2 kcal/mol into the equation ln K = –G/RT].
2. “Non-Zaitsev” Products Can Dominate When Sufficient Steric Hindrance Is Present
The use of a bulky base for the elimination reaction may often result in “non-Zaitsev” products. The use of sodium or potassium t-butoxide (KOt-Bu) is a classic example, as is the use of lithium di-isopropyl amide (LDA). The theory is that the bulky base can react more rapidly with the proton on a beta-carbon that is least sterically hindered, resulting in the formation of the least substituted alkene. [See Bulky Bases in Elimination Reactions for more information].
These "non-Zaitsev" products are often referred to as "Hofmann products." What is the reason for this?
3. The “Hofmann Degradation”
There were few methods for analysing complex molecules available in 1851. Degradation was one technique for determining the composition of an unknown compound by breaking it down into smaller parts and looking for clues in the fragments. August Wilhelm von Hofmann invented a two-step amine degradation process that was later named after him.
The first step is to treat an amine with a large amount of methyl iodide [CH3I], which produces an ammonium salt.
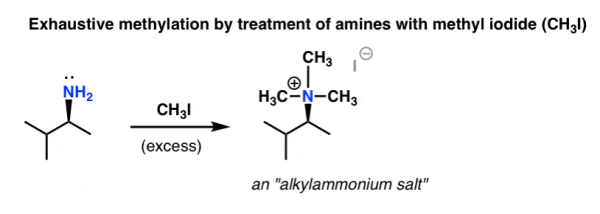
Q10) Write a note on Hofmann-elimination.
A10)
Hofmann elimination is the method of producing tertiary amines and alkenes from quaternary ammonium, as previously stated. Exhaustive methylation is another name for this procedure. When it comes to asymmetrical amines, the Hofmann rule states that the main alkene product is the least substituted and least stable. The Hofmann elimination method is named after its discoverer, August Wilhelm Von Hofmann, a German chemist. The Hofmann removal is depicted in the diagram below.
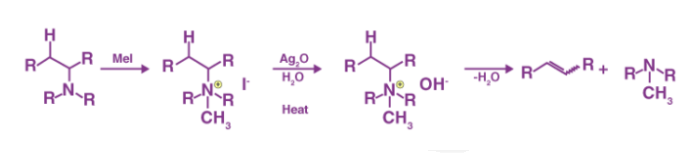
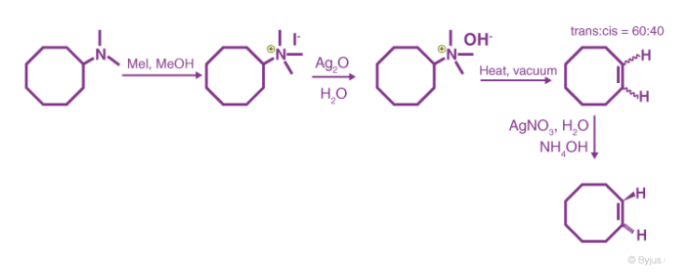
It's worth noting that methyl iodide is overused because it lacks beta hydrogens and hence cannot compete in the elimination reaction. The alkene isomer with the least substituted double bond is formed as the major product if the given alkyl group contains two separate sets of beta hydrogens that are available for the elimination process. The synthesis of trans-cyclooctene, shown below, is an interesting example of the Hofmann elimination process or exhaustive methylation process.
It's worth noting that the neutral NR3 group outperforms the anionic NH2– and NR2– groups as a leaving group. Steric effects caused by large leaving groups also have an impact on the reaction's outcome.
Hofmann Elimination Mechanism
The exhaustive methylation mechanism, also known as the Hofmann elimination mechanism, begins with the amine attacking the methyl iodide salt with a beta-hydrogen to form the ammonium iodide salt. Silver iodide is formed when the iodide reacts with the silver oxide. Since this silver iodide is insoluble, it precipitates out of solution. The water is deprotonated by a silver oxide ion, which forms a hydroxide ion. This mixture is currently being heated. The removal reaction is aided by the sun, and the hydroxide now picks up the beta-hydrogen from the ammonium ion. It also produces an amine, which is used to make the final alkene product. The following steps can be used to demonstrate the mechanism:
Step 1: As shown below, the ammonium iodide salt is formed by treating an amine with a beta-hydrogen or quaternary ammonium with excess methyl iodide.
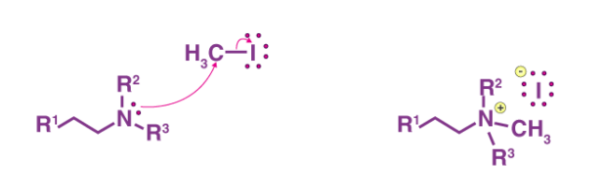
Step 2 The iodide ion is substituted for the hydroxide ion by a reaction between the iodide and silver oxide, followed by deprotonation of water by the silver oxide ion. This stage is depicted in the diagram below.
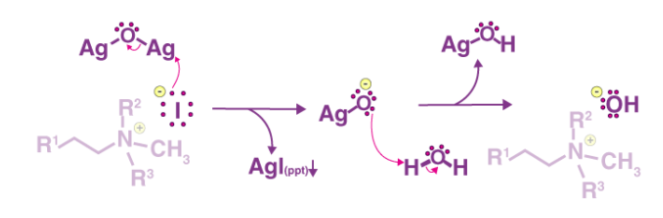
Step 3
The mixture is heated to facilitate and elimination reaction and form the required product.
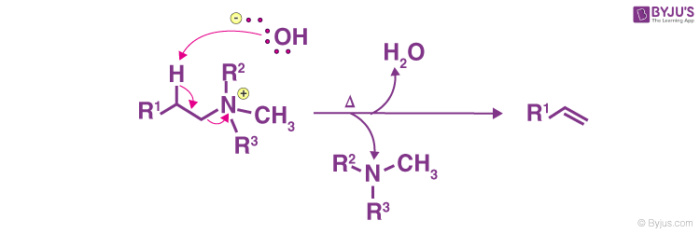
Thus, the required olefin product is achieved. The tertiary amine product is also formed. The Hofmann elimination process can be categorized as an elimination reaction as well as an olefination reaction.
Q11) What is Hinsberg Reagent?
A11)
Benzene sulfonyl chloride is also used as Hinsberg reagent. This name comes from its use in the Hinsberg test, which is used to detect and distinguish primary, secondary, and tertiary amines in a sample.
An organosulfur compound is what this reagent is. C6H5SO2Cl is the chemical formula for this substance. Hinsberg reagent has the appearance of a colourless oil that is viscous in nature and soluble in organic solvents.
This Reagent reacts with compounds that contain reactive O-H and N-H bonds. It is used to make sulfonamides and sulfonamide esters (by reacting with amines) (via reaction with alcohol).
Preparation of Hinsberg Reagent
The needed reagent is obtained by chlorinating benzene sulfonic acid or benzene sulfonic acid salts with phosphorus oxychloride (POCl3).
Reacting benzene with chloro sulfuric acid is another way to make the appropriate Hinsberg Reagent (chemical formula HSO3Cl). Both of these methods for preparing the necessary reagent are shown in the diagram below.
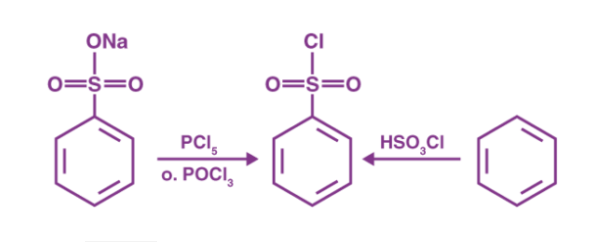
Hinsberg Test
The Hinsberg test is a chemical reaction that determines whether an amine is primary, secondary, or tertiary. Oscar Heinrich Daniel Hinsberg, a German chemist, was the first to describe this reaction in 1890.
The amines behave as nucleophiles in the Hinsberg Test, attacking the electrophile (sulfonyl chloride). This causes the chloride to be displaced, resulting in the formation of sulfonamides. As primary and secondary amines combine to form sulfonamides, the resulting sulfonamide is insoluble and precipitates as a solid from the solution.
Hinsberg Reaction Pathways
As benzene sulfonyl chloride reacts with primary amines, a sulfonamide compound is formed that is alkali soluble. The following diagram depicts this reaction.
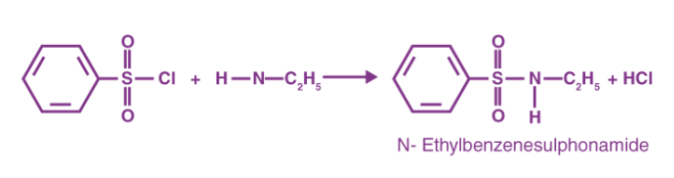
As benzene sulfonyl chloride reacts with secondary amines, a sulfonamide compound is formed that is not alkali soluble. Below is an example of this form of reaction.
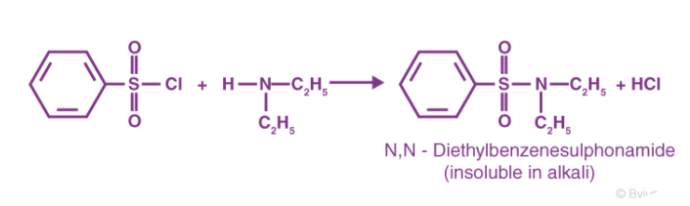
Between a tertiary amine and the benzene sulfonyl chloride reagent, no such reaction occurs. Sulfonyl chloride hydrolysis is aided by tertiary amines. This reaction produces salts that are water soluble.
As a result, the Hinsberg reagent reacts differently with principal, secondary, and tertiary amines. These variations can be seen in the sulfonamide product's alkali solubility.
Q12) Explain Bonding of nitrogen.
A12)
The positive charge is delocalized over the two nitrogen atoms, according to a resonance description of diazonium ions. Nucleophiles cannot bind to the inner nitrogen, but negative nucleophiles will bond (or couple) to the terminal nitrogen to produce neutral azo compounds. This coupling to the terminal nitrogen should be relatively quick and reversible, as seen in the following equation. E / Z stereoisomers of the azo products can exist. In fact, the E-isomer is found to predominate at equilibrium.

Reversal to the diazonium ion and slow nucleophilic substitution at carbon (with irreversible nitrogen loss) would be the ultimate course of reaction unless these azo products are trapped or stabilised in some way, as stated in the previous section. As phenyldiazonium bisufate is quickly added to a cold sodium hydroxide solution, a relatively stable solution of sodium phenyldiazoate (the conjugate base of the initially formed diazoic acid) results. Lowering the pH of this solution produces phenyldiazoic acid (pKa ca. 7), which dissociates back to the diazonium ion and then undergoes substitution to produce phenol.
C6H5N2(+) HSO4(–) + NaOH (cold solution) |  | C6H5N2–OH + NaOH (cold) |  | C6H5N2–O(–) Na(+) |
Phenyldiazonium bisulfate |
| Phenyldiazoic acid |
| Sodium phenyldiazoate |
Aryl diazonium salts may be reduced to the corresponding hydrazines by mild reducing agents such as sodium bisulfite, stannous chloride or zinc dust. The bisulfite reduction may proceed by an initial sulfur-nitrogen coupling, as shown in the following equation.
| NaHSO3  |
| NaHSO3  |
| H2O  |
|
Electrophilic aromatic substitution of activated benzene derivatives by diazonium electrophiles is the most common application of diazo coupling reactions. These reactions produce highly coloured aromatic azo compounds, which are used as synthetic dyestuffs and are known as azo dyes. The colour of azobenzene (Y=Z=H) is light orange; however, depending on the nature of the aromatic rings and the substituents they bear, the colour of other azo compounds can vary from red to deep blue. Although there are cis/trans isomer pairs of azo compounds, the majority of well-characterized and stable compounds are trans.

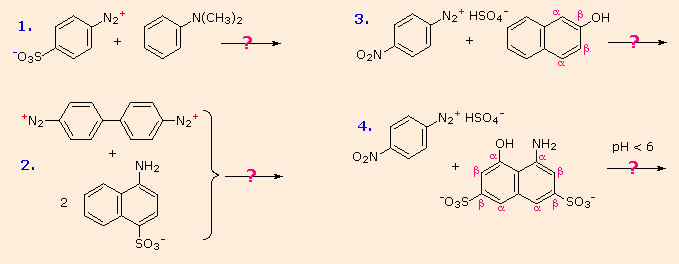
The following are some examples of azo coupling reactions. A few basic rules will help predict how certain reactions will unfold:
I An amino group is a better activating substituent than a hydroxyl group at acid pH (6). (i.e., a phenol). Due to increased phenoxide base concentration, phenolic functions are stronger activators at alkaline pH (> 7.5).
If that position is open, coupling to an activated benzene ring occurs preferentially para to the activating group. If this does not happen, ortho-coupling will occur.
(iii) Naphthalene undergoes electrophilic substitution at alpha sites more quickly than at beta sites, but ortho-coupling is favoured. Examples of / notation in naphthalene’s can be found in the diagram.
Q13) Give the Substitution and Elimination Reactions of Amines.
A13)
In nucleophilic substitution or base-catalyzed elimination reactions, amino functions are rarely used as leaving groups. Indeed, they are even less powerful than hydroxyl and alkoxyl groups in this regard. Modifying the leaving group (OH (–) or OR (–) to increase its stability as an anion was a useful technique for increasing the reactivity of the oxygen function in alcohols and ethers (or equivalent). The power of the corresponding conjugate acids can be used to measure this stability.
As previously mentioned, 1o and 2o-amines are much weaker acids than alcohols, so it's not shocking that forcing the nitrogen mechanism to act as a nucleophilic leaving group is difficult. Heating an amine with HBr or HI, for example, does not usually result in the formation of the corresponding alkyl halide, as it does with alcohols and ethers. The acidity of the putative ammonium leaving group is at least ten powers of ten lower than that of an equivalent oxonium species in this sense. Due to the extreme stability of this leaving group, nitrogen loss from diazonium intermediates is a significant exception in this relation (the conjugate acid of N2 would be an extraordinarily strong acid).
The tetra alkyl (4o-) ammonium salts are one class of amine derivatives that has proven useful in SN2 and E2 reactions. The majority of applications involving this class of compounds are eliminations, although there have been a few reports of SN2 substitution.
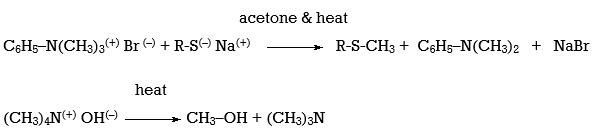
Q14) Explain Hofmann Elimination.
A14)
Hofmann eliminations are 4o-ammonium salt reduction reactions. Since the counter anion in most 4o-ammonium salts is halide, it is frequently replaced by the more simple hydroxide ion via silver hydroxide reaction (or silver oxide). To impact the E2-like removal of a 3o-amine, the resulting hydroxide salt must be heated (100 - 200 oC). A typical Hofmann elimination is shown in Example #1 below. As previously mentioned, when looking at alkyl halide elimination reactions, one of the alkyl substituents on nitrogen must have one or more beta-hydrogens for an elimination to occur.
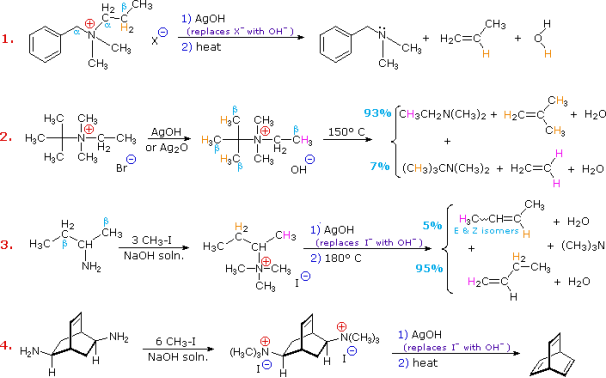
Two of the alkyl substituents on nitrogen in Example #2 have beta-hydrogens, all of which are on methyl groups (colored orange & magenta). The alkene with the more strongly substituted double bond is the main product of the removal, reflecting not only the 3:1 numerical advantage of certain beta-hydrogens, but also the greater stability of the double bond.
Example #3 highlights two main aspects of the Hofmann elimination:
First, by extensive alkylation, typically with methyl iodide, simple amines are easily converted to the necessary 4o-ammonium salts (methyl has no beta-hydrogens and cannot compete in the elimination reaction). In example #4, exhaustive methylation is demonstrated once more.
Second, when an alkyl group has two sets of beta-hydrogens available for removal (coloured orange and magenta here), the alkene isomer with the less substituted double bond is always the main product.
The Hofmann Rule refers to the tendency of Hofmann eliminations to produce the less-substituted double bond isomer, which contrasts sharply with the Zaitsev Rule for dehydrohalogenations and dehydrations. The Hofmann Rule does not apply when other triggering groups, such as phenyl or carbonyl, are present. Thus, if 2-amino-1-phenylpropane is handled in the manner of example #3, the result consists largely of 1-phenylpropene (E & Z-isomers) (E & Z-isomers).
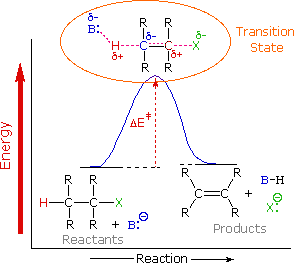
Example #2 exemplifies a key feature of the Hofmann elimination. If the nitrogen atom is part of a ring, this removal technique does not extract the nitrogen as a separate 3o-amine product in a single application. A second Hofmann elimination is needed to separate the nitrogen feature from the molecule. Indeed, if the nitrogen atom was part of two rings (fused or spiro), it would take three Hofmann eliminations to separate the nitrogen from the remaining molecular structure.
The less stable trans-cyclooctene is the main product in Example #3, which is followed by the cis-isomer. Since the cis-cycloalkene will be produced by an anti-E2-transition state, the trans-isomer must be produced by a syn-elimination. A syn-elimination may also generate the cis-cyclooctene released in this reaction. The structure of cyclooctane is conformationally complex. There are a number of puckered conformations that escape angle strain, and one of the most stable is shown on the right. Many of these conformers have several eclipsed bonds, and transannular hydrogen crowding is inevitable. Since the trimethylammonium substituent is wide (roughly the same size as tert-butyl), it will most likely take on an equatorial orientation to prevent steric crowding. An axial-like orientation of this bulky group is likely needed for an anti-E2 transition state, making this an unfavourable direction.
Q15) Explain the Reaction of Aryl Diazonium salts.
A15)
The salts of aryl diazonium are essential intermediates. They're made in a cold (between 0 and 10 oC) aqueous solution and react with nucleophiles to release nitrogen. The following diagram depicts some of the most widely used substitution reactions. These reactions are energetically preferred because the leaving group (N2) is thermodynamically very stable. Sandmeyer reactions are those substitution reactions that are catalysed by cuprous salts. Treatment with BF4(–) causes fluoride substitution, which is known as the Schiemann reaction. It is possible to isolate stable diazonium tetrafluoroborate salts, which lose nitrogen when heated to produce an arylfluoride product. The reductive elimination of an amino (or nitro) group is achieved in the top reaction with hypophosphorus acid, H3PO2. This reduction, unlike nucleophilic substitution reactions, is most likely the product of a radical process.
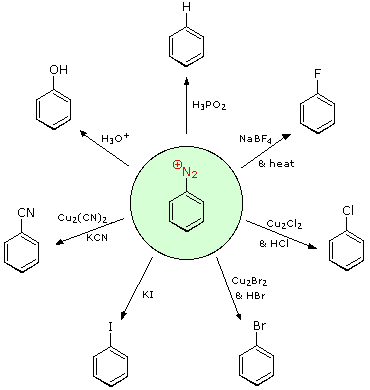
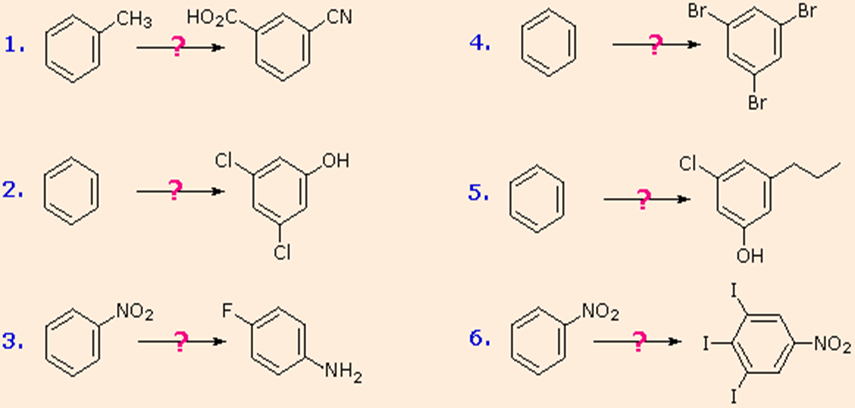
These aryl diazonium substitution reactions vastly increase the number of poly substituted benzene derivatives that can be synthesised. Think of the following possibilities:
I The corresponding nitro compound is the most common precursor to an aryl amine. An aromatic ring is deactivated by a nitro substituent, which guides electrophilic substitution to meta locations.
(ii) There are many methods for reducing a nitro group to an amine. An aromatic ring is strongly activated by the amine substituent, which guides electrophilic substitution to ortho and para positions.
(iii) By forming an amide derivative (reversible), the activating character of an amine substituent may be reduced, or even modified to deactivating and meta-directing by forming a quaternary-ammonium salt (irreversible).
(iv) By converting an aryl amine to a diazonium ion intermediate, a number of different groups (including hydrogen) can be substituted, which can then be used in subsequent reactions.