Unit – 2
Diazonium Salts and Polynuclear Hydrocarbons
Q1) Explain Diazonium salts.
A1)
The diazonium salts (di means "two," azo means "nitrogen," and ium means "cationic"), or diazonium compounds, are a class of organic compounds with the general formula RN2+X, where X is an organic or inorganic anion (for example, Cl–, Br–, BF4–, and so on) and R is an alkyl or aryl group.
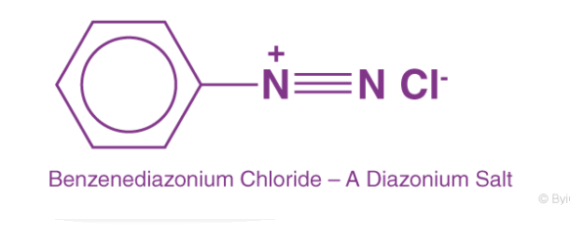
As a result, they have two nitrogen atoms, one of which is charged. The diazonium salts include benzene diazonium chloride (C6H5N2+Cl–), benzene diazonium hydrogen sulphate (C6H5N2+HSO4–), and others.
Diazonium salts are one of the most versatile organic-inorganic combinations available. RN+2X is a general way of representing it. The R stands for an organic group, usually an aryl group, and the X stands for ion. Cl–, Br–, and BF4 are the most common X in diazonium salts. The existence of the N+2 group or the diazonium group gives these salts their names.
The suffix diazonium is added to the parent hydrocarbon from which these salts are formed, and then the anion X, such as bromide, is added after that.
The value of these compounds can be found in their applications and applications.
Diazonium Salts Preparation
- Diazotization or dissociation is the method of converting an organic compound, typically primary aromatic amines, into diazonium salts.
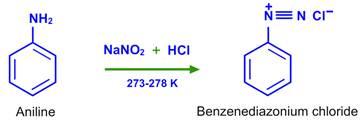
- The diazonium group is extremely unstable under normal conditions, so it is rarely stored; instead, it is usually used immediately after preparation. One of the most popular methods for preparing diazonium salt is to react nitrous acid with aromatic amines. Benzene diazonium chloride is formed when aniline (aromatic amine) reacts with nitrous acid to form a diazonium salt.
- Since nitrous acid is a highly toxic gas, it is usually made in situ (during the reaction) by reacting NaNO2 with a mineral acid.
- Temperature is another important factor that influences product formation during the preparation of these salts; most diazonium salts are stable below 5° C.
- As a result, it's critical to keep the reaction temperature below 5°C, or else the diazonium group will decompose into N2 as soon as it's produced.
- The reaction of aniline with NaNO2 and hydrochloric acid is shown below, with benzene diazonium chloride as the end product.
- C6H5NH2+NaNO2+2HCl C6H5NH2+NaNO2+2HCl
- 0–5 degrees Celsius
- CC6H5N+Cl+NaCl+2H2O CC6H5N+Cl+NaCl+2H2O
- The diazonium group has a wide range of applications. This mixture of organic and inorganic components has been a blessing for scientists in a variety of fields, from dyes and pigments to the synthesis of different organic compounds.
Q2) Give the properties and importance of Diazonium Salts.
A2)
Properties of Diazonium Salts
1. They have an ionic nature.
2. They are soluble in water.
3. Aryl diazonium salts are crystalline solids that are colourless.
4. Benzene diazonium chloride is water soluble but only reacts with it when heated.
5. Water does not dissolve benzene diazonium fluoroborate. At room temperature, it is somewhat stable.
Importance of Diazonium Salts
- They're used in the dye and pigment industries to make dyed fabrics, and they're often used in record replication because of their ability to break down near ultraviolet light.
- They can be used to make a wide range of organic compounds, especially aryl derivatives
- For the preparation of aryl iodides and fluorides, direct halogenation is not an option. It is impossible to substitute a cyano group for the nucleophilic replacement of chlorine in chlorobenzene. Diazonium salts, on the other hand, can easily be used to make cyan benzene.
- Direct substitution of benzene cannot be used to make substituted aromatic compounds. We make these compounds by replacing the diazo group in diazonium salts.
- They're used to introduce –F, –Br, –Cl, –I, –NO2, –OH, and –CN groups into the aromatic ring as intermediates.
Q3) State the general formula of Diazonium Salts?
A3)
Where X stands for Cl, Br, HSO4, BF4, etc.
Aryl diazonium salts are used in the synthesis of a wide range of organic compounds as intermediates.
The ions of primary alkyl diazonium are not very stable. When dry, they decompose quickly and can be explosive.
At low temperatures, aryl diazonium salts are only stable for a short time. By delocalizing the positive charge around the aromatic ring, resonance structures help to stabilize the ion.
Aniline and nitrous acid are used to make aryl diazonium salts, which are made in situ (freshly prepared before use) at 273-278 K.
Ex:
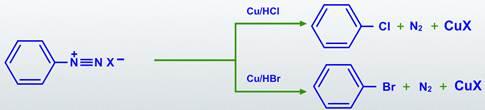
In the presence of copper (I) ions, diazonium groups are substituted by chloride, bromide, or cyanide in the Sandmeyer reactions.
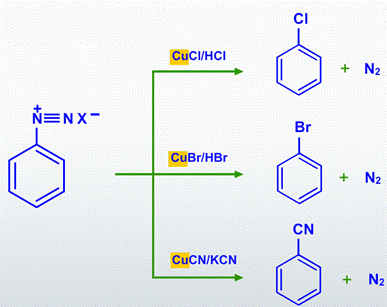
By treating the diazonium salt solution with halo acid in the presence of copper powder, diazonium groups are substituted with Chlorine (or) Bromine in the Gattermann reactions.
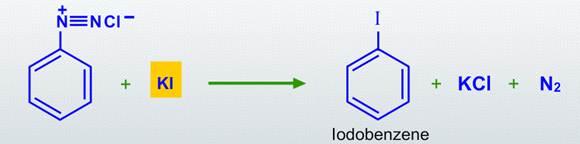
Treatment with potassium iodide will replace the dizonium group with iodine.
A mechanism for producing aryl fluorides is the Schiemann reaction.
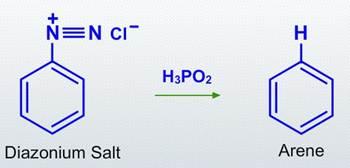
After treatment with mild reducing agents such as hypo phosphorous acid (or) ethanol, the diazonium group is replaced by hydrogen in reductive deamination.
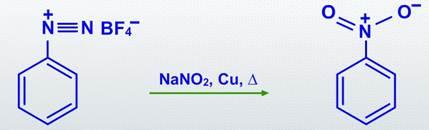
The diazonium group is substituted by a -NO2 group in nitration reactions.
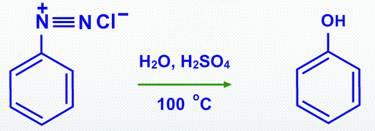
By hydrolyzing diazonium salts with dilute sulphuric acid and heating, phenols can be produced.
As diazonium salts react with phenol (or) aniline, azo coupling reactions occur.
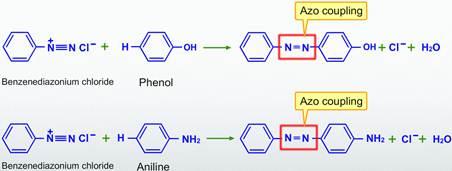
Q4) Explain preparation and their synthetic application of Diazonium salts.
A4)
Preparation and their synthetic application.
Ethers
Ethers are organic compounds in which two alkyl or aryl groups are joined by an oxygen atom. The general formula for these is R-O-R', where R and R' can be alkyl or aryl groups.
Consider the following scenario:
Classification of Ethers
Ethers are divided into two types based on the similarity or dissimilarity of alkyl or aryl groups attached to the central oxygen atom:
- Simple or Symmetrical ethers:
When R = R', the ether is referred to as simple or symmetrical ether. Consider the following scenario:
CH3CH2OCH2CH3
Diethyl ether
2. Mixed or Unsymmetrical ethers:
If R R', ether is referred to as mixed or unsymmetrical ether. Consider the following scenario:
C6H5OC2H5
Ethylphenyl ether
Q5) Write short note on Nomenclature of Ethers.
A5)
Common naming system:
To begin, locate the alkyl or aryl groups that are attached to the central oxygen atom.
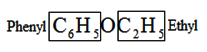
Then alphabetize the alkyl or aryl groups and add the suffix ether.
C6H5OC2H5
Ethylphenyl ether
IUPAC naming system:
Ethers are classified as ‘alkoxy alkanes' in the JUPAC scheme, in which the ethereal oxygen (to which the two alkyl or aryl groups are attached) is taken along with the smaller alkyl group, whereas the larger alkyl group is considered a part of the alkane.
The oxygen atom is sp3 hybridized, with two bond pairs and two lone pairs of electrons arranged in a roughly tetrahedral configuration. The ROR' bond angle is greater than the 109° tetrahedral angle. The interaction between the two bulky (–R) groups is the reason for this. The C–O bond length (141 pm) is nearly identical to that of alcohols.
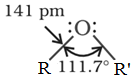
Q6) Explain Preparation of Ethers.
A6)
The following are some general ether preparation methods:
(a) By dehydration of 1° alcohols (SN2 reaction):
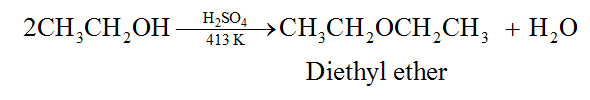
This method can be used to make ethers that only contain primary alkyl groups.
Dehydration of 2° and 3° alcohols yields alkenes but not ethers.
(b) By Williamson's synthesis (SN2 reaction):
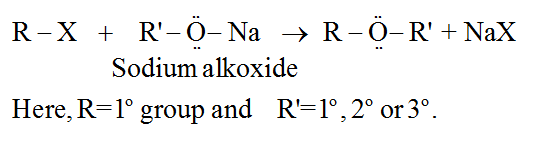
For example:
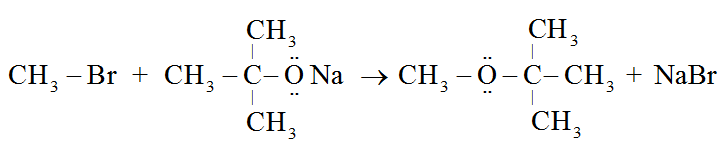
This process can also be used to make ethers with substituted alkyl groups (2° or 3°).
Alkyl halides (RX), on the other hand, produce only alkenes as a main product.
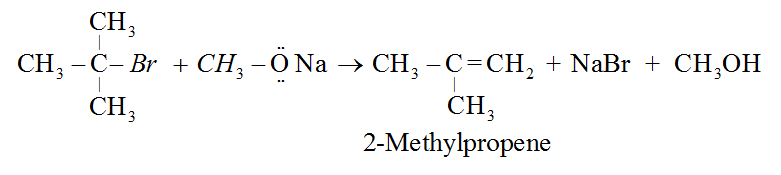
Q7) Explain Physical and Chemical properties of Ethers.
A7)
- Even if the two R and R' groups are identical, ethers are polar in nature and have a net dipole moment. This is due to the fact that their form is twisted.
- B. Ethers have a lower boiling point than alcohols due to their inability to form hydrogen bonds between molecules.
- Lower ethers (those with three carbon atoms or less) are totally miscible in water. As the number of carbon atoms increases, the solubility of ethers decreases.
- D. Ethers serve as both Bronsted and Lewis bases due to the presence of electron-donating R groups and the lone pair on oxygen atoms.
Chemical properties of Ethers
(a) Reactions of ethers involving C−O bond cleavage
Reaction with hydrogen halides (HX):
For symmetrical ethers:
R ‒ O ‒ R + HX → 2RX + H2O
For asymmetrical ethers:
R ‒ O ‒ R’ + HX → RX + R’‒OH
For a given ether, the reactivity of hydrogen halides follows the order:
HCl < HBr < HI
For aromatic ethers:
Due to the stability of the aryl-oxygen bond, the reaction with HI causes the cleavage of the RO bond in aromatic ether.
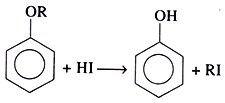
The halide formed is a tertiary halide when one of the alkyl groups is a tertiary group. This is because a more stable 3° carbonium ion forms.
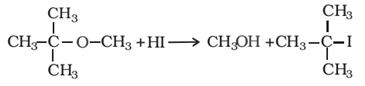
(b) Electrophilic substitution
The benzene ring's alkoxy group (-OR) is o- and p-directing, activating the aromatic ring for electrophilic substitution.
Aromatic ethers undergo a number of significant electrophilic substitution reactions.
• Halogenation:
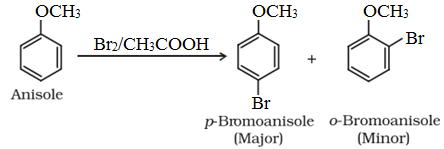
• Nitration:
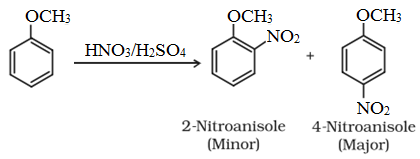
• Friedel-Crafts reaction:
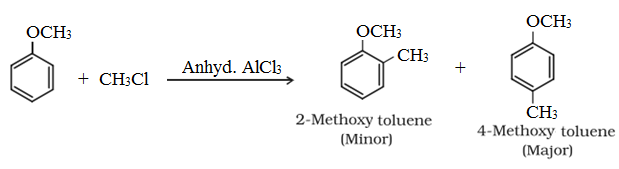
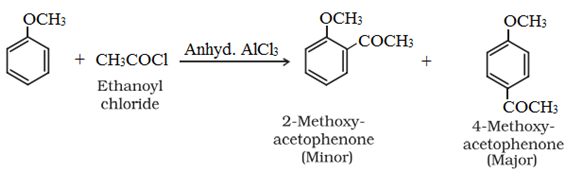
• Uses of Ethers
1. At low temperatures, dimethyl ether is used as a refrigerant and a solvent.
2. Diethyl ether is used in surgery as an anesthetic.
3. Diethyl ether is used as a solvent for oils, gums, and resins, among other things.
4. Because of its high boiling point, phenyl ether is used as a heat transfer medium.
Q8) Explain the types of rubber.
A8)
Types of rubber
Natural rubber and synthetic rubber are the two most common forms of rubber.
Natural rubber
These are the elastomers that come from nature. Natural rubber is a mixture of solid particles suspended in a milky white liquid called latex that drips from the bark of tropical and subtropical trees. Brazil, India, Indonesia, Malaysia, and Sri Lanka are the largest producers of latex rubber. It is known as cis- 1, 4- polyisoprene and is produced by polymerizing isoprene (2 methyl-1, 3-butadiene) with the chemical formula (C5H8) n. They are rendered by loosely linking the monomers of isoprene (C5H8) in the form of a long-tangled chain, in simple terms.
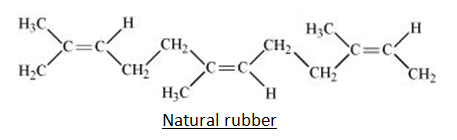
Q9) Write short note on Preparation of Natural Rubber.
A9)
- Rubber tapping – A small V-cut on the tree bark is used to extract the milky white liquid latex from the rubber trees in a cup. To congeal the rubber particles, the collected latex is washed, filtered, and reacted with acids.
- Mastication – The rubber produced via the tapping process is still unfit for usage. When it is cold, it is brittle, but once warmed up, it becomes very gluey. The rubber is allowed to pass through the rollers and pressed to soften to make it more durable to work with, removing its fragile nature and strong odour. This process is repeated depending on the rubber's necessary properties. Extra chemical additives are also applied during this process to improve the properties of rubber.
- Calendering is a rubber shaping method that uses rollers to give the rubber its shape (after proper mixing of the chemical ingredients).
- The finished product is then extruded into hollow tubes using a special extrusion system with specially built holes.
- Vulcanization – Performing any of the steps above would not result in rubber that is solid or hard enough to be used in car tyres or machinery. Sulphur is applied to the rubber, which is then heated between 373 and 415 degrees Celsius to improve both of these properties. Vulcanization is the term for this process. Sulphur serves as a cross-linking agent, and rubber becomes cross-linked and hard after vulcanization.
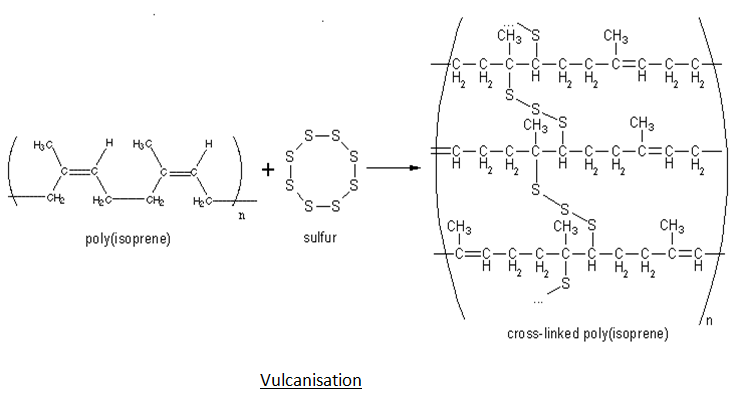
Q10) Explain Synthetic rubbers with its Preparation and Uses.
A10)
Petroleum and natural gas are used to make synthetic rubbers. Polymerization of 1, 3 – butadiene derivatives or copolymerization of 1, 3 – butadiene with unsaturated monomer yields it.
Preparation of synthetic rubbers:
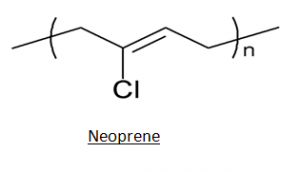
1. Polychloroprene (Neoprene): – It's a chloroprene polymer that's made by joining chloroprene monomers together.
1. Buna – N: It is a 1, 3 butadiene and acrylonitrile copolymer that is formed in the presence of a peroxide catalyst.
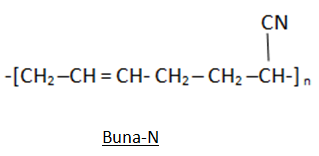
Uses of Rubber
- Rubber can be used for a variety of things and on a variety of platforms, some of which are mentioned below.
- It's used to line chutes, containers, and industrial mixers, among other things. It can be used as a strong insulator due to its water-proof and durable properties.
- It can be used as wetsuits and expandable garments in the clothing industry, such as gym and cycling shorts.
- Rubbers are also used for flooring because they provide insulation, reduce fatigue, and are waterproof and slip-resistant.
- Its use in the car industry can be seen in tyres, brake padding, airbags, seats, and roofs, among other places.
Q11) What is Polynuclear Hydrocarbons?
A11)
Reactions of naphthalene and anthracene Structure:
Haworth synthesis of naphthalene
- The simplest polycyclic aromatic hydrocarbon is naphthalene (C10H8), which is an organic compound. It's a crystalline white solid with a distinct odour. The structure of naphthalene is made up of a fused pair of benzene rings. It's well known for being the primary ingredient in mothballs. Haworth synthesis can be used to make it; the steps are as follows:
3 benzoyl propionic acid is produced by the Friedel craft acylation reaction of benzene with succinic anhydride.
2. The Clemmenson reaction with 3-benzoylpropionic acid yields 4-phenylebutanoic acid in the second step.
3. When this compound is heated in the presence of concentrated sulphuric acid, the water molecule is eliminated, resulting in the formation of a ring structure of -tetralene.
4. Tetrahydronaphthalene is produced by the Clemmenson reaction of -tetralene.
5. Tetrahydronaphthalene is dehydrogenated in the presence of selenium to yield naphthalene.
Q12) What are Aromatic Hydrocarbons?
A12)
Aromatic hydrocarbons are organic compounds with a circular structure and sigma bonds as well as delocalized pi electrons. Arenes or aryl hydrocarbons are other names for them.
The following are a few examples of aromatic hydrocarbons. All of these compounds have a benzene ring, as can be shown.
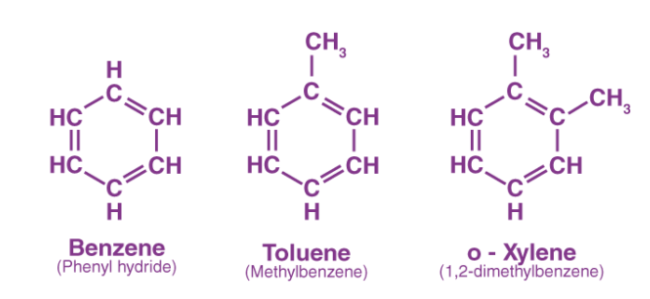
Check ⇒ Xylene
• Heteroarenes are aromatic hydrocarbons that don't have a benzene ring in their structure. Huckel's law (total number of pi electrons in a monocyclic ring = 4n + 2 where n is either positive integer or zero) applies to all of these heteroarenes.
• A minimum of one carbon is substituted by either nitrogen, oxygen, or sulphur in these compounds. Furan (which contains oxygen) and pyridine are two examples of heteroarenes (contains nitrogen).
Q13) Explain the Properties of Aromatic Hydrocarbons and Its Reactions.
A13)
“The first compound that was categorized as an aromatic hydrocarbon was benzene”. Aromatic hydrocarbons that lack a benzene ring in their structure are known as heteroarenes. All of these heteroarenes follow Huckel's law (total number of pi electrons in a monocyclic ring = 4n + 2, where n is a positive integer or zero).
In these compounds, a minimum of one carbon is replaced by nitrogen, oxygen, or sulphur. Heteroarenes include furan (which contains oxygen) and pyridine (contains nitrogen).
Reactions of Aromatic Hydrocarbons
Aromatic hydrocarbons are used as the main reactant in many organic chemical reactions. This subsection includes a list of such reactions, as well as a brief summary of each of them.
1. Aromatic Substitution Reactions
• One substituent on the ring of an aromatic hydrocarbon, usually a hydrogen atom, is replaced by a separate substituent group in these reactions.
• The following are examples of aromatic substitution reactions:
• Aromatic nucleophilic substitution reactions
• Aromatic electrophilic substitution reactions
• Nucleophilic aromatic substitution reactions with radical nucleophiles
• The electrophilic substitution seen in the nitration reaction of salicylic acid is an example of an aromatic substitution reaction.
2. Coupling Reactions
The coupling of two radical fragments is accomplished with the aid of a metal catalyst in these types of reactions. The following types of bonds can form when aromatic hydrocarbons undergo coupling reactions.
Carbon-carbon bonds can be formed in coupling reactions of arenes, resulting in products such as vinyl arenes, alkyl arenes, and so on. Carbon-oxygen bonds can also be formed in these reactions, resulting in aryloxy compounds.
In coupling reactions, carbon-nitrogen bonds may form, resulting in products like aniline.
The arylation of perfluoro benzenes, as shown below, is an example of a coupling reaction involving aromatic hydrocarbons.
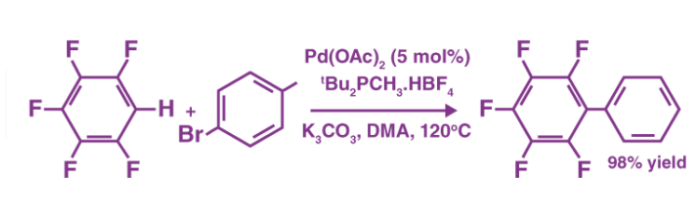
Palladium (II) acetate is the catalyst in this reaction. It's also worth noting that DMA stands for Dimethylacetamide.
Hydrogenation Reactions
• Arenes hydrogenation reactions typically result in the formation of saturated rings. The reduction of 1-naphthol into a mixture of different isomers of decalin-ol is an example of such a reaction.
• The hydrogenation of resorcinol with the aid of spongy nickel (also known as Raney nickel) and aqueous NaOH is another example of such a reaction. This reaction starts with the formation of an enolate, which is then alkylated (with methyl iodide) to produce 2-methyl-1,3-cyclohexanedione.
Q14) Explain the Uses of Aromatic Hydrocarbons.
A14)
Aromatic hydrocarbons are widely used in biological and synthetic processes. The following is a list of some of the many applications for aromatic hydrocarbons.
1. Chlorophyll, a green pigment found in plants, is made up of aromatic hydrocarbons and plays an important role in the processing of food in plants.
2. These aromatic hydrocarbons are also found in nucleic acids and amino acids in the human body.
3. In model glues, methylbenzene, an aromatic hydrocarbon, is used as a solvent.
4. Naphthalene is a key ingredient in the manufacture of mothballs.
5. Phenanthrene, an aryl hydrocarbon, is used in the production of medicines, dyes, and explosives.
6. TNT (trinitrotoluene) is a very important aromatic hydrocarbon that is commonly used in explosives.
7. Aromatic hydrocarbons are widely used in the plastic and petrochemical industries. Polycyclic Aromatic Hydrocarbons
There are hydrocarbons that are made up of fused aromatic rings. Coal, tar, oil, and some cooked foods like smoked fish, burnt bread, and so on contain these.
Naphthalene is a typical example of these polycyclic hydrocarbons. These substances are referred to as contaminants. Methylbenzene, Naphthalene, Phenanthrene, Trinitrotoluene, and o-dihydroxybenzene are examples of aromatic hydrocarbons.
Q15) Give introduction about Benzene and Explain its Discovery.
A15)
• With the chemical formula C6H6, benzene is one of the most essential organic compounds. The parent compound of all aromatic compounds is benzene.
• The simplest organic aromatic hydrocarbon is benzene. Benzene is a natural constituent of crude oil and one of the most basic petrochemicals. It is a colourless liquid with a gasoline-like odour. In nature, benzene is extremely toxic and carcinogenic. Polystyrene is the most popular use for it.
• Benzene is a naturally occurring compound that is found in many plants and animals and is formed by volcanoes and forest fires, but it is also a major industrial chemical manufactured from coal and oil. Benzene is a smooth, colourless liquid when it is pure. In manufacturing, benzene is used to produce a variety of products, including plastics, detergents, and pesticides. It can also be used in gasoline.
Discovery of Benzene
• The word benzene comes from the gum benzoin, which is also known as "Benjamin." Gum benzoin was previously thought to be an aromatic resin. Benzene was first found in illumination gas by Michael Faraday, an English physicist. Mitscherich, a German chemist, coined the term benzene in 1833. The cyclic structure of benzene was unknown until 1865, when German professor August Kekule deduced it from a dream in which he saw a snake chewing its own tail.
• Kekule, on the other hand, did not find any interactions between the double bonds. Linus Pauling, an American chemist, suggested that benzene had a hybrid form of delocalized electrons. Kekule's discovery had been refined in this way. While benzene has a nice, sweet aroma, it is carcinogenic.
Q16) Describe Benzene Characteristics.
A16
)
• The English physicist Michael Faraday discovered benzene in 1825, and it was made widely available in 1842 after it was discovered to contain benzene. Benzene is now produced in large quantities from petroleum.
• Benzene is a colourless liquid with the formula C6H6 and a distinct odour. Benzene is a closed ring of six carbon atoms linked by single and double bonds that alternate. A single hydrogen atom binds each carbon atom. Benzene melts at 5.5 degrees Celsius and boils at 80.1 degrees Celsius. Aromatic compounds, which include benzene and its derivatives, are an important chemical group. Medicines, plastics, gasoline, synthetic rubber, and dyes all use benzene as a precursor.
• As a result, benzene hydrogenation occurs much more slowly than that of other organic compounds with carbon-carbon double bonds, and benzene is much more difficult to oxidise than alkenes. The majority of benzene reactions fall into the category of electrophilic aromatic substitution, which keeps the ring intact when replacing one of the hydrogens attached to it. These reactions are extremely flexible and are often used to make benzene derivatives.
Q17) Describe the Structure of Benzene and its Properties.
A17)
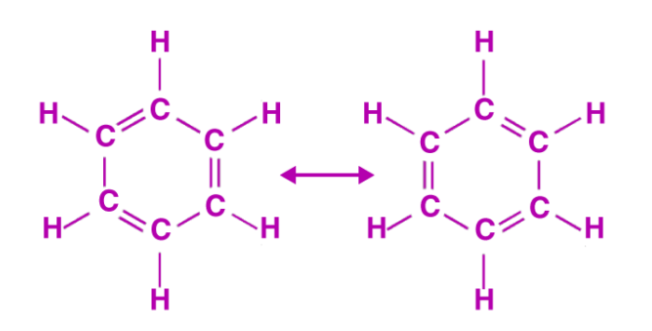
Since its discovery, the structure of benzene has piqued researchers' interest. Each carbon atom in benzene is arranged in a six-membered ring and is bonded to just one hydrogen atom, making it a cyclic hydrocarbon (chemical formula: C6H6). The formation of three delocalized – orbitals spanning all six carbon atoms is described by molecular orbital theory, while valence bond theory defines two stable resonance structures for the ring.
Properties of Benzene
The following are some of benzene's properties:
1. Benzene is water insoluble, but it is soluble in organic solvents.
2. It's a colourless liquid with a pleasant fragrance.
3. It is lighter than water, with a density of 0.87g cm-3
4. Benzene has a high melting point and a low boiling point. (Boiling temperature: 80.5°C, melting temperature: 5.5°C)
5. There is resonance in benzene.
6. It burns with a sooty flame and is extremely flammable.
Q18) Describe Resonance of Benzene.
A18)
According to valence bond theory, the oscillating double bonds in the benzene ring are explained using resonance structures. The benzene ring's carbon atoms are all sp2 hybridised. Six C-C sigma bonds are formed when one of one atom's two sp2 hybridised orbitals overlaps with the sp2 orbital of an adjacent carbon atom. Six C-H sigma bonds are formed when other left sp2 hybridised orbitals combine with hydrogen's s orbital. By lateral overlap, the remaining unhybridized p orbitals of carbon atoms form bonds with neighbouring carbon atoms.
This explains why C1 – C2, C3 – C4, C5 – C6 bonds or C2 – C3, C4 – C5, C6-C1 bonds are equally likely to form. In the figure below, a circle is inserted into the ring to represent the hybrid structure. As a result, it describes the formation of two Kekule-proposed resonance structures.
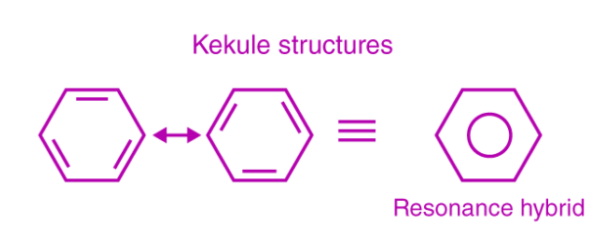
Aromaticity of benzene
Since the C-C bonds formed in the ring are not exactly single or double, but rather of intermediate length, benzene is classified as an aromatic compound. Aromatic compounds are classified as benzenoids (those with a benzene ring) or non-benzenoids (those without a benzene ring) if they obey the Huckel law. According to Huckel's law, an aromatic ring must possess the following characteristics:
1. The definition of planarity
2. Complete delocalization of the ring's electrons
3. The ring contains (4n + 2) electrons, where n is an integer (n = 0, 1, 2, etc.).
Q19) Explain the Uses of Benzene and Health Effect of Benzene.
A19)
Benzene is used in a wide range of industrial processes, including the production of lubricants, fabrics, rubbers, dyes, and synthetic fibres. However, since benzene is toxic and carcinogenic, it has limited non-industrial applications. The various applications of Benzene are mentioned below.
1. The chemical benzene is used to make phenol. It's also used to make aniline, which is used in dyes, and dodecylbenzene, which is used in detergents.
2. In the past, benzene was used to degrease metal.
3. It is used in the production of nylon fibres.
4. The primary use of benzene is in the production of other chemicals such as ethylbenzene, cyclohexane, cumene, nitrobenzene, and alkylbenzene.
Health Effects of Benzene
Acute benzene exposure is harmful to the central nervous system; however, the myelotoxic and potential chromosome damaging and leukemogenic effects of benzene must be considered when evaluating the chronic effects. The length of time it takes for chlorine benzene toxicity to manifest suggests a wide range of individual susceptibility.
The majority of cases of extreme benzene poisoning have been identified in employees who were exposed to high levels of benzene in unsanitary working conditions. All cases of "leukimia associated with benzene toxicity" are most likely the result of exposure to relatively high concentrations of benzene and other chemicals.