Unit - 4
Alkaloids and Terpenes
Q1) Explain Natural Occurrence of Alkaloids.
A1)
Many natural compounds can be found in nature. Alkaloids appear to be unique among the several groups of naturally occurring organic compounds, including carbohydrates, lipids, proteins, amino acids, anthocyanins, flavonoids, and steroids. What distinguishes them? They're made up of amino acids and can be synthesized by plants and animals as secondary metabolites. In living organisms, these compounds play an important role. Alkaloids have long been thought to be extremely valuable to humans, despite the fact that they are secondary metabolites, which may mean that they are ineffective. In relatively small doses, alkaloids had strong biological effects on animal and human species. Alkaloids are used in a variety of foods and beverages, as well as in stimulant medications. Anti-inflammatory, anticancer, analgesics, local anaesthetic and pain reliever, neuropharmacologic, antimicrobial, antifungal, and a variety of other behaviours were demonstrated. Alkaloids are useful in medicine and other aspects of human life as diet foods, vitamins, and pharmaceuticals. Alkaloids are also essential compounds in organic synthesis for the development of new semisynthetic and synthetic compounds with potentially higher biological activity than their parent compounds.
Since alkaloids have such a wide structural diversity, there is no single method for extracting them from natural raw materials. Most approaches take advantage of the fact that most alkaloids are soluble in organic solvents but not in water, and that their salts have the opposite tendency.
Alkaloids are found in almost all plants. Their mixture is extracted first, followed by the separation of individual alkaloids. Until extraction, the plants are thoroughly ground. The majority of alkaloids are found in raw plants as organic acid salts. The alkaloids extracted can remain salts or transform into bases. The alkaloid bases are extracted using organic solvents such as 1,2-dichloroethane, chloroform, diethyl ether, or benzene after the raw material has been processed with alkaline solutions. Weak acids dissolve the impurities, converting alkaloid bases to salts that can be washed away with water. An aqueous solution of alkaloid salts is rendered alkaline again, if possible, then treated with an organic solvent. The procedure is repeated until the desired purity level is reached.
The raw plant material is treated by a poor acidic solution in acidic extraction (e.g., acetic acid in water, ethanol, or methanol). The alkaloids are then converted to basic forms, which are extracted using organic solvents (if the extraction was performed with alcohol, it is removed first, and the remainder is dissolved in water). As previously mentioned, the solution is filtered.
Alkaloids are isolated from their mixtures using varying solvent solubility and reactivity with different reagents, or by distillation.
Researchers have reported a variety of alkaloids in insects, with solenopsins, which are alkaloids found in the venom of fire ants, receiving the most attention. Solvent immersion of live fire ants or centrifugation of live ants accompanied by silica-gel chromatography purification are both effective methods for extracting insect alkaloids. Based on their absorbance peak around 232 nanometers, tracking and dosing the extracted solenopsin ant alkaloids has been identified as possible.
Q2) Explain Flavonoids.
A2)
Flavonoids are a wide group of polyphenolic compounds with a benzo-pyrone structure that are found in plants. The phenylpropanoid pathway is used to make them. According to research, secondary phenolic metabolites, such as flavonoids, are responsible for a wide range of pharmacological activities. Flavonoids are hydroxylated phenolic compounds that plants are known to produce in response to microbial infection. 3. Their actions are governed by a set of rules. Flavonoids' chemical properties are determined by their structural class, degree of hydroxylation, other substitutions and conjugations, and polymerization degree. 4. The potential health benefits derived from the antioxidant activities of these polyphenolic compounds have sparked renewed interest in these compounds. Flavonoids' functional hydroxyl groups scavenge free radicals and/or chelate metal ions to mediate their antioxidant effects. 5. And 6. Metal chelation may be crucial in preventing the generation of radicals that damage target biomolecules. 7, 8, 9. Flavonoids are thought to have health-promoting properties as a dietary ingredient due to their high antioxidant potential in both vivo and in vitro systems. 9, ten. Flavonoids have the ability to activate defensive enzyme systems in humans. Flavonoids have been shown to protect against a variety of infectious (bacterial and viral diseases) and degenerative diseases such as cardiovascular disease, cancer, and other age-related diseases in a number of studies 2, 9, 10. In this study, the processes involved in flavonoids' defence are discussed separately. Flavonoids also serve as a secondary antioxidant protection mechanism in plant tissues that have been subjected to various abiotic and biotic stresses. Flavonoids are found in the nuclei of mesophyll cells as well as ROS production centres. They also control plant growth factors including auxin 11. Biosynthetic genes have been assembled in a variety of bacteria and fungi to boost flavonoid production 12. The structural aspects of flavonoids and their protective functions against a variety of human diseases are discussed in this study. Flavonoids' functions in plants, as well as their microbial production, have been identified.
Q3) Explain Chemistry of Flavonoids
A3)
Flavonoids are a class of natural compounds found in plants with varying phenolic structures. Oranges were used to isolate a new drug in 1930. Vitamin P was given to it because it was thought to be a part of a new class of vitamins at the time. It was later discovered that this material was a flavonoid (rutin), and over 4000 different flavonoids have been known to date 13.
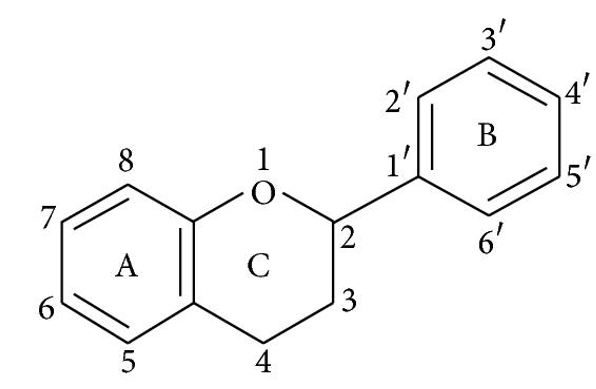
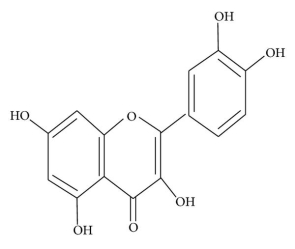
Flavonoids have a fifteen-carbon skeleton, which is made up of two benzene rings (A and B in Figure 1) connected by a heterocyclic pyrane ring (C). Flavones (e.g., flavone, apigenin, and luteolin), flavonols (e.g., quercetin, kaempferol, myricetin, and fisetin), flavanones (e.g., flavanone, hesperetin, and naringenin), and others are only a few examples. Table 1 depicts their general systems. Individual flavonoids within a class differ in their degree of oxidation and substitution pattern of the C ring, whereas individual compounds within a class differ in their substitution pattern of the A and B rings 13.
Structure of flavonoids.
Flavonoids come in a variety of forms, including aglycones, glycosides, and methylated derivatives. The aglycone structure is the most fundamental flavonoid the framework (Figure 1). A -pyrone (flavonols and flavanones) or its dihydroderivative is a condensed six-member ring with the benzene ring (flavonols and flavanones). Flavonoids (2-position) and isoflavonoids (2-position) are divided by the position of the benzenoid substituent (3-position). Flavonols differ from flavanones in that they have a 3-position hydroxyl group and a C2–C3 double bond 40. The positions 3, 5, 7, 2, 3′, 4′, and 5′ are often hydroxylated in flavonoids. The alcohol group's methyl ethers and acetyl esters are known to exist in nature. The glycosidic linkage is usually found in positions 3 or 7 when glycosides are formed, and the carbohydrate may be L-rhamnose, D-glucose, glucorhamnose, galactose, or arabinose 41.
Q4) Explain Spectral Characteristics of Flavonoids
A4)
Spectroscopy studies on flavonoids have shown that the majority of flavones and flavonols have two big absorption bands: The B ring absorption is represented by Band I (320–385 nm), while the A ring absorption is represented by Band II (250–285 nm). Functional groups attached to the flavonoid skeleton can cause absorption to change from 367 nm in kaempferol (3,5,7,4′-hydroxyl groups) to 371 nm in quercetin (3,5,7,3′,4′-hydroxyl groups) and 374 nm in myricetin (3,5,7,3′,4′,5′-hydroxyl groups) 42. Flavones are distinguished from flavonols by the absence of a 3-hydroxyl group. According to their UV spectral characteristics, flavanones have a saturated heterocyclic C ring with no conjugation between the A and B rings. 42. Flavanones have a high Band II absorption maximum between 270 and 295 nm, respectively 288 nm (naringenin) and 285 nm (taxifolin), with just a shoulder at 326 and 327 nm for Band I. When a monosubstituted B ring is present, Band II appears as a single peak (270 nm), but when a di-, tri-, or o-substituted B ring is present, it appears as two peaks or one peak (258 nm) with a shoulder (272 nm). Since anthocyanins have a distinct Band I peak in the 450–560 nm region due to the hydroxyl cinnamoyl system of the B ring and a Band II peak in the 240–280 nm region due to the benzoyl system of the A ring, their colour varies with the number and location of the hydroxyl groups 44.
Q5) Write short note on Flavonoid Rich Food and Medicinal Plants
A5)
Flavonoids are the most common and widely distributed group of plant phenolic compounds, with flavonoids found in virtually all plant parts, particularly photosynthesizing plant cells. They are a big component of flowering plants' colouring. Flavonoids are a necessary component of both human and animal diets. Table 2 lists several food sources that contain various flavonoids groups. Flavonoids cannot be synthesised by humans or animals because they are phytochemicals 45. As a result, flavonoids present in animals are derived from plants rather than being biosynthesized in the wild. The most common flavonoids in foods are flavonols. Flavonoids in food are responsible for colour, flavour, fat oxidation prevention, and vitamin and enzyme safety 46. Soy isoflavones, flavonols, and flavones are the flavonoids present in the largest concentrations in the human diet. While catechins are found in most fruits and some legumes, their concentrations range from 4.5 to 610 mg/kg 47. Depending on the methods used, food preparation and processing may reduce flavonoid levels. Orange juices, for example, were found to contain 81–200 mg/L soluble flavanones in a recent study, while cloud content was 206–644 mg/L, implying that flavanones are concentrated in the cloud during processing and storage 48. Because of the large variety of available flavonoids and their widespread distribution in different plants, as well as the complex ingestion of flavonoids in humans, accurate estimate of the average dietary intake of flavonoids is difficult 49.
There has recently been a wave of interest in medicinal plants' therapeutic potential, which may be due to their phenolic compounds, specifically flavonoids 50, 51. Humans have been consuming flavonoids for around 4 million years, since the beginning of human life on the planet. They have a wide range of biological properties that benefit human health and help to minimize disease risk. During atherosclerosis, oxidative alteration of LDL cholesterol is thought to play a key role. Glabridin, a major polyphenolic compound contained in the Glycyrrhiza glabra (Fabaceae) plant, inhibits LDL oxidation via a process involving free radical scavenging. 51. Several epidemiological studies have suggested that drinking green or black tea may lower blood cholesterol levels and blood pressure, thereby reducing the risk of cardiovascular disease. Flavonoids, as flavorants, colourants, and antioxidants, are known to affect the consistency and stability of foods. 53 and 54. Flavonoids found in berries have been shown to benefit people with Parkinson's disease and enhance memory in the elderly. In hypertensive rats 55, the total flavonoid fraction of Astragalus complanatus had an antihypertensive impact. Consumption of antioxidant flavonoids has been shown to be inversely linked to the risk of dementia 56. Table 3 lists some of the medicinal plants that are high in flavonoids.
The therapeutic effectiveness of flavonoids can be influenced by their solubility. With the exception of an occasional instance of allergy, the poor solubility of flavonoid aglycones in water, combined with their limited residence period in the intestine and lower absorption, humans do not experience acute toxic effects from flavonoid intake. The low solubility of flavonoids in water makes their medicinal applications difficult. As a result, semisynthetic, water-soluble flavonoids such as hydroxyethylrutosides and inositol-2-phosphatequercetin have been linked to the treatment of hypertension and microbleeding 57.
Q6) Write short note on Metabolism of Flavonoids in Humans.
A6)
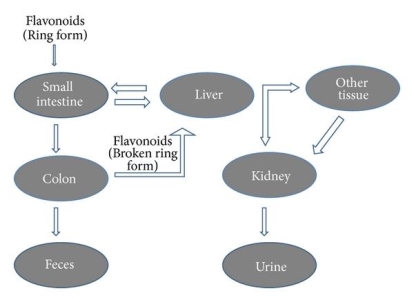
The physicochemical properties of dietary flavonoids liberated from food by chewing can influence their absorption, including molecular size, configuration, lipophilicity, solubility, and pKa. The flavonoid may be absorbed directly from the small intestine or may first pass through the colon. It could be determined by the flavonoid's composition, specifically whether it is a glycoside or an aglycone. Except for the catechin subclass, most flavonoids are found in plants bound to sugars as b-glycosides (Figure 2). Flavonoid glycosides must be converted into aglycan type 58 before they can be consumed by the small intestine.
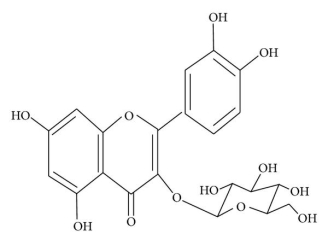
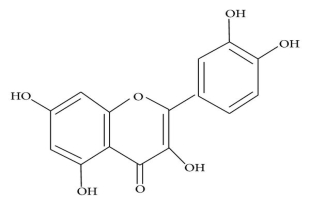
Q7) Explain the Structure of
(a) flavonoid glycoside and
(b) aglycone flavonoid.
A7)
The intestinal Na+-dependent glucose cotransporter (SGLT1) 58 transports hydrophilic flavonoid glucosides like quercetin through the small intestine. Another theory is that flavonoid glucosides are hydrolyzed by lactase phloridzin hydrolase (LPH), a -glucosidase found on the outside of the small intestine's brush boundary membrane. The liberated aglycone can then be absorbed by the small intestine 59. This LPH enzyme's substrate specificity differs greatly across a wide variety of flavonoids 60 glycosides (glucosides, galactosides, arabinosides, xylosides, and rhamnosides). The glycosides that aren't substrates for these enzymes are transported to the colon, where bacteria can hydrolyze flavonoid glycosides while simultaneously degrading the liberated flavonoid aglycones 61. Since the colon's absorption potential is far lower than that of the small intestine, only a minor amount of these glycosides can be absorbed.
After absorption, flavonoids are glucuronidated, sulfated, or methylated in the liver, or metabolised to smaller phenolic compounds 62. Except for catechins 63, no free flavonoid aglycones can be contained in plasma or urine as a result of these conjugation reactions. The bioavailability of these flavonoids varies significantly depending on the food source; for example, quercetin absorption from onions is four times greater than that from apple or tea 64. Intestinal microflora degrades flavonoids secreted with bile in the intestine and those that cannot be absorbed from the small intestine in the colon, as well as the flavonoid ring structure (Figure 3). Under the influence of acidic conditions in the stomach, oligomeric flavonoids can be hydrolyzed into monomers and dimers. Larger molecules make it to the intestine, where bacteria degrade them. Flavonoid glycosides' bioavailability is largely determined by their sugar moiety. Bioavailability has been shown to be reduced by dimerization. Isoflavones have the maximum bioavailability of all the flavonoids subclasses 65. The flavonoid content of green tea is rapidly absorbed, as shown by their elevated levels in plasma and urine. Soon after ingestion, they penetrate the systemic circulation and induce a substantial rise in plasma antioxidant status 66.
Q8) Explain the Biological Activities of Flavonoids
A8)
- Antioxidant Activity
Flavonoids have a variety of biochemical properties, but the ability to act as antioxidants is the property that almost any group of flavonoids is best known for. The arrangement of functional groups around the nuclear structure determines flavonoids' antioxidant activity. Several mechanisms of antioxidant activity, such as radical scavenging and metal ion chelation, are heavily influenced by the configuration, substitution, and total number of hydroxyl groups. 67, 4, Since it donates hydrogen and an electron to hydroxyl, peroxyl, and peroxynitrite radicals, stabilising them and giving rise to a relatively stable flavonoids radical 68, the B ring hydroxyl configuration is the most important determinant of scavenging of ROS and RNS.
(1) suppression of ROS formation (either by inhibiting enzymes or chelating trace elements involved in free radical generation); (2) scavenging ROS; and (3) upregulation or preservation of antioxidant defences are some of the mechanisms of antioxidant action. 69 and 70. The majority of the mechanisms described above are involved in flavonoid action. Any of the results mediated by them may be the product of a combination of radical scavenging activity and enzyme functions interacting. Microsomal monooxygenase, glutathione S-transferase, mitochondrial succinoxidase, NADH oxidase, and other ROS-producing enzymes are all inhibited by flavonoids 71.
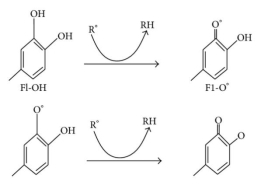
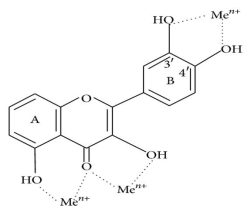
Oxidative stress causes lipid peroxidation, which is a common side effect. Flavonoids protect lipids from oxidative damage in a variety of ways. 51, 5, By reducing hydrogen peroxide and generating the highly reactive hydroxyl radical, free metal ions boost ROS production. Flavonoids (Fl-OH) can reduce strongly oxidising free radicals (redox potentials in the range 2.13–1.0 V) such as superoxide, peroxyl, alkoxyl, and hydroxyl radicals by hydrogen atom donation due to their lower redox potentials (Figure 4(a)). Flavonoids inhibit free radical production due to their ability to chelate metal ions (iron, copper, etc.) 70, 72. Iron-chelating and iron-stabilizing properties of quercetin are well-known. Trace metals bind to unique locations in flavonoid structures' rings 73. Figure 4 depicts the binding points (b).
(a)
(b)
Q9) Explain the Hepatoprotective Activity of Flavonoids
A9)
Several flavonoids have been found to have hapatoprotective properties, including catechin, apigenin, quercetin, naringenin, rutin, and venoruton 81. Hepatic clinical symptoms may evolve as a result of a variety of chronic diseases, such as diabetes. In the liver of diabetic mice, glutamate-cysteine ligase catalytic subunit (Gclc) expression, glutathione, and reactive oxygen species (ROS) levels were found to be lower. Anthocyanins are gaining popularity due to their ability to protect against a variety of diseases. According to Zhu et al. 82, the anthocyanin cyanidin-3-O-glucoside (C3G) increases hepatic Gclc expression by raising cAMP levels, which cause protein kinase A (PKA), which then upregulates CREB phosphorylation, promoting CREB-DNA binding and increasing Gclc transcription. Hepatic ROS levels and proapoptotic signalling are reduced when Gclc expression is increased. C3G therapy also reduces hepatic lipid peroxidation, prevents the release of proinflammatory cytokines, and protects against hepatic steatosis 82.
Silymarin is a flavonoid derived from the seeds and fruit of the milk thistle Silybum marianum with three structural components: silibinin, silydianine, and silychristine (Compositae). Silymarin has been shown to induce DNA-dependent RNA polymerase 1 enzymatic activity and subsequent RNA and protein biosynthesis, resulting in DNA biosynthesis and cell proliferation, which leads to liver regeneration only in damaged livers 83. In normal livers, silymarin increases proliferating hepatocytes in response to FB1 (Fumonisin B1, a mycotoxin developed by Fusarium verticillioides) induced cell death while having no effect on cell proliferation. Silymarin's pharmacological properties include cell membrane permeability and integrity control, leukotriene inhibition, ROS scavenging, suppression of NF-B function, protein kinase depression, and collagen development 84. Cirrhosis, ischemic injury, and toxic hepatitis caused by various toxins such as acetaminophen and toxic mushroom 85 can all be treated with silymarin.
Flavonoids isolated from Laggera alata were found to have hepatoprotective properties against carbon tetrachloride (CCl4)-induced injury in primary cultured neonatal rat hepatocytes and in rats with hepatic harm. Flavonoids at concentrations ranging from 1 to 100 g/mL increased cell viability and prevented CCl4 86-induced hepatocyte aspartate aminotransferase (AST) and alanine aminotransferase (ALT) leakage. In an in vivo study, flavonoids at 50, 100, and 200 mg/kg oral doses significantly decreased serum levels of AST, ALT, total protein, and albumin, as well as liver levels of hydroxyproline and sialic acid. Flavonoid 86 therapy also improved the weakened liver, according to histopathological tests.
Flavonoids have been shown to be effective and secure in the treatment of hepatobiliary dysfunction and digestive symptoms such as fullness, lack of appetite, nausea, and abdominal pain in several clinical studies. In HepG2 cells, flavonoids from Equisetum arvense, as well as hirustrin and avicularin isolated from other sources, have been shown to protect against chemically induced hepatotoxicity 87, 88.
Q10) Explain the Role of Flavonoids in Plants
A10)
- Combating Oxidative Stress
Plants have long been known to use flavonoids for a variety of purposes 140. Various abiotic and biotic factors contribute to the development of reactive oxygen species (ROS) in plants, resulting in oxidative stress. Plants' flavonoid biosynthesis is almost entirely boosted by oxidative stress. They have the ability to absorb the most energetic solar wavelengths (UV-B and UV-A), inhibit ROS development, and quench ROS once they have developed 141. When early plants transferred from the water to the land, flavonoids performed primary UV-B screening functions. The nature of substitution on different rings of flavonoids determines the extent of antioxidant potential and ability to absorb UV-wavelengths. Flavonoids with a dihydroxy B ring substituted have a higher antioxidant potential, whereas monohydroxy B ring substituted flavonoids have a higher ability to absorb UV-wavelengths 141.
Flavonoids' most volatile hydroxyl groups (7-OH in flavones and 3-OH in flavonols) are typically glycosylated. Glycosylation increases solubility in aqueous cellular environments, protects reactive hydroxyl groups from autooxidation 142, and enables flavonoids to be transported from the endoplasmic reticulum to different cellular compartments and secreted to the plasma membrane and cell wall 143. Antioxidant flavonoids have recently been discovered in the nucleus of mesophyll cells and inside ROS-generating centres, such as the chloroplast. H2O2, hydroxyl radicals, and singlet oxygen 141, 144 can all be easily quenched here.
2. As Growth Regulator
Flavonoids play an important role in plant-environment interactions. Auxin movement and catabolism can be controlled by flavonoids (in the nanomolar range). Flavonoids' ability to generate auxin gradients results in phenotypes with various morphoanatomical characteristics 150. Controlling auxin movement with flavonoids may be extremely useful in stress-induced morphogenic responses in plants, such as the flight strategy of sessile organisms in unfavourable environments 151. When comparing dihydroxy flavonoids-rich species to monohydroxy flavonoids-rich species, phenotypes with remarkably different morphological traits emerge 152. In sunny environments, dwarf bushy phenotypes with few, thin, and dense leaves to direct sunlight irradiance are common, shielding leaves deep in the canopy from light-induced cellular homeostasis perturbations. Shaded plants, on the other hand, have long internodes and broad leaf lamina with reduced leaf thickness 151, and are rich in kaempferol and/or apigenin derivatives (with negligible concentrations of quercetin derivatives).
Q11) Explain the Microbial Production of Flavonoids.
A11)
In response to the low efficiency of plant and chemical synthesis, researchers have turned to microorganisms to produce flavonoids using metabolic engineering and synthetic biology 156. The chemical synthesis of flavonoids necessitates harsh reaction conditions and the use of hazardous chemicals 157. Combinatorial biosynthesis provides an advantage for the processing of unusual and costly natural products due to rapid advancements in molecular biology techniques and the influx of genome knowledge from a variety of species. It can be used in both simple and complex transformations without the time-consuming blocking and deblocking steps that organic synthesis needs 158. Flavonoids have been developed using a variety of prokaryotes and eukaryotes, including E. Coli, Saccharomyces cerevisiae, Streptomyces venezuelae, and Phellinus igniarius, a medicinal mushroom 12.
- Phenylpropanoid Pathway
The precursor for a large number of flavonoids formed by the phenylpropanoid synthetic pathway in plants is naringenin chalcone. Fermentative synthesis of flavanones from the amino acid precursors phenylalanine and tyrosine 159 in E. Coli carrying an artificially assembled phenylpropanoid pathway was the first example of a nearly complete biosynthetic pathway in plants being formed in a heterologous microorganism. The action of phenylalanine ammonia-lyase deaminates phenylalanine to yield cinnamic acid, which is the first step in the phenylpropanoid pathway in plants (PAL). Cinnamate-4-hydroxylase (C4H) converts cinnamic acid to p-coumaric acid, which is then activated to p-coumararoyl-CoA by 4-coumarate: CoA ligase. Chalcone synthase (CHS) catalyses the condensation of three acetate units from malonyl-CoA with p-coumaroyl-CoA to produce naringenin chalcone in a stepwise fashion. In vitro 160, chalcone isomerase (CHI) or nonenzymatically converts naringenin chalcone to naringenin.
2. Enhancement of Flavonoid Production
The primary molecular biology technology procedures used in the heterologous development of flavonoids are the combination of promoter and target genes, knockout of related genes, overexpression of malonyl-CoA, and construction of artificial P450 enzymes. Every phenylpropanoid pathway gene is cloned in the host under the guidance of the promoter, which frequently plays a key role in the heterologous expression of secondary metabolites. T7, ermE*, and GAL1 promoter 12 have all been used to increase flavonoid development in response to the needs of particular hosts. One of the disadvantages in the microbiological processing of flavonoids was the exceptionally low concentration of malonyl-CoA in the microbial cell.
Advantages
The use of phytochemicals, especially flavonoids, in the prevention and treatment of diseases is well known. Flavonoids are found naturally in fruits and vegetables. The physical, chemical, and physiological properties of various flavonoids found in nature vary. Flavonoids' structure-function relationship epitomises major biological activities. Many flavonoids have been shown to have antibacterial, hepatoprotective, anti-inflammatory, anticancer, and antiviral properties. In developed countries, these substances are more widely used. Biochemical methods must be used to confirm the therapeutic application of new substances. Flavonoids can now be produced in large quantities thanks to genetic modifications. More accomplishments would contribute to newer discoveries and, without a doubt, a new era of flavonoid-based pharmaceutical agents for the treatment of many infectious and degenerative diseases.
Q12) Give Assessment of Antioxidant capacity.
A12)
For this purpose, a considerable number of chemical assays, coupled with highly sensitive, quick and usually automated detection technologies, have been developed (Table) 1,7,8,9,10,11,12,13,14,15,16,17,18,19. These assays claim to provide a quick, easy, convenient, and reliable in vitro AC determination by providing different information about the ROS–sample interaction. They typically use a chemical system that includes an oxidant (free radicals or other ROS), an oxidizable probe (not needed for certain assays), and the antioxidants being studied.
Table:
List of the widespread assays to evaluate Antioxidant Capacity.
Assay | Main Mechanism | Oxidant | Probe | Detection | Ref. |
ORAC | HAT | ROO∙ | FL | Fluorescence | 1,7 |
DPPH | SET | DPPH∙ | DPPH∙ | Absorbance | 8,9 |
FRAP | SET | Fe3+ | Fe(TPTZ)22+ | Absorbance | 10 |
TEAC | SET | ABTS∙+ | ABTS+ | Absorbance | 11,12 |
HORAC | HAT | HO∙ | FL | Fluorescence | 13 |
TRAP | HAT | ROO∙ | β-PE | Fluorescence | 14,15 |
CUPRAC | SET | Cu2+ | Neocuproine | Absorbance | 16 |
Total Phenolic Assay | SET | FCR | FCR | Absorbance | 17 |
Crocin Bleaching | HAT | ROO∙ | Crocin | Absorbance | 18 |
Chemiluminescence | HAT | H2O2 | Luminol | Fluorescence | 19 |
Q13) Explain Methods Based on the Use of the Soybean Lipoxygenase-1 Isoenzyme.
A13)
Two advanced assays focused on the use of the soybean lipoxygenase (LOX)-1 isoenzyme as a device to produce different physiological radicals and to highlight different antioxidant mechanisms have been developed in order to develop novel methodologies/approaches capable of providing AC values as closely as possible resembling the in vivo response. They were given the names LOX/RNO 25 and LOX–FL 26 because they are based on the reactions of LOX-1 isoenzyme with 4-nitroso-N, N-dimethylaniline (RNO) 27 and LOX-1 isoenzyme with fluorescein (FL). The first LOX/RNO approach was used to investigate the antioxidant properties of a variety of natural products, including food-grade antioxidants, cereal and pseudocereal grains, grain-derived products, and fruits (28,29,30,31,32). The LOX–FL assay was recently established 26 by combining the advantages of the LOX/RNO process, which stem from the use of soybean LOX-1 isoform, with the high sensitivity of the ORAC assay, which stems from the use of FL as a probe 33. In comparison to the LOX/RNO process, this has the added benefit of being a technique that can be used for both in vitro measurements of food extracts and ex vivo analysis of human blood samples. As a result, it has primarily been used to calculate ex vivo AC in human blood after both short-term 34 and long-term 35 dietary antioxidant intakes.
The data obtained from in vitro and ex vivo measurements using LOX-1-based assays is provided in an up-to-date summary.
Q14) Explain the Effects of different antioxidant compounds on LOX/RNO and LOX–FL reactions.
A14)
Compound | Ki a or IC50 b | Antioxidant/Trolox c |
LOX/RNO Reaction | ||
Trolox | 7.0 ± 1.1 mM a,d | 1.00 |
Resveratrol | 1.7 ± 0.2 mM a,d | 4.57 ± 0.33 e |
Ferulic acid | 10.7 ± 2.1 mM a,d | 0.99 ± 0.11e |
Gallic acid | 6.7 ± 1.3 mM a,d | 0.61 ± 0.05 e |
Apigenin | 3.4 ± 0.5 mM a,d | 1.49 ± 0.09 e |
Catechin | 13.6 ± 1.8 mM a,d | 0.42 ± 0.03 e |
l-Ascorbic acid | 14.0 ± 1.9 mM a,d | 0.83 ± 0.09 e |
Glutathione | 19.7 ± 3.2 mM b,d | n.d. |
α-tocopherol | 1.1 ± 0.1 mM a,d | 3.43 ± 0.25 e |
β-carotene | 7.8 ± 0.9 μM b,d | n.d. |
LOX–FL Reaction | ||
Trolox | 5 ± 0.6 μM a; 26 ± 2 μM b,d | 1.00 |
Albumin | 25 ± 2 μM b,d | 1.04 ± 0.08 e,f |
Bilirubin | 7.6 ± 0.6 μM b,d | 3.43 ± 0.28 e,f |
l-Ascorbic acid | 1360 ± 10 μM b,d | 0.02 ± 0.002 e,f |
Uric acid | 40 ± 2 μM b,d | 0.66 ± 0.04 e,f |
a Ki values were obtained by measuring reaction rates at various RNO, FL, and antioxidant concentrations; b IC50 values represent the antioxidant concentration capable of halving the rates of LOX/RNO (or LOX-mediated oxodiene generation in the case of glutathione) or LOX–FL reactions; c ratio between the gradient of the plot reporting the decrease of the rate (%) of LOX/RNO or LOX–FL reactions; d mean value minus standard deviation (n = 4); e mean value minus standard error (n = 4); f unpublished data; n.d. Pastore et al. 25 and Soccio et al. 26 were used as inspiration.
Trolox, a noncompetitive and competitive inhibitor of LOX/RNO and LOX–FL reactions with Ki values of about 7 mM and 5 M, respectively, was discovered among different antioxidant compounds 25,26. In addition, for the LOX–FL reaction, an IC50 of 26 M was discovered. With the exception of -carotene, the LOX/RNO reaction was found to be less sensitive to single pure antioxidants than LOX–FL, as indicated by millimolar and micromolar Ki or IC50 values obtained, respectively. All other antioxidants studied showed a linear dependency of inhibition (percent) of the reaction rate on compound concentration, similar to Trolox. In light of this, the efficacy of each compound in relation to Trolox was calculated (Table, column Antioxidant/Trolox) by dividing the gradient of the plot relative to the antioxidant by the gradient of the plot relative to Trolox. Resveratrol, apigenin, and -tocopherol had higher LOX/RNO activity than Trolox, ferulic and L-ascorbic acids had comparable activity, and gallic acid and catechin had lower activity. In the LOX–FL reaction, bilirubin was more active than Trolox, uric and L-ascorbic acids had lower activity, and albumin had equal activity.
Q15) Explain the Evaluation of Human Blood Antioxidant Status after Food Intake.
A15)
● Antioxidant/Oxidant Balance (AOB) Approach and its Application in Short-Term Studies
It should be noted that in vitro AC testing may only allow for merceological characterization of plant food commodities and cannot be linked to biological effects of dietary bioactive compounds after ingestion 7,21. In reality, in vitro AC analysis of food extracts does not provide information on dietary phytochemical bioavailability, that is, the fraction of ingested antioxidant compound that reaches the systemic circulation, as well as in vivo stability, tissue retention, and in situ reactivity. The United States Department of Agriculture (USDA) removed its ORAC database for selected foods from its nutrient data laboratory website 22 in 2012 as a result of these factors.
A new method has been suggested to gain more physiologically meaningful knowledge about potential health effects of foods by combining in vitro AC examination of foods with an ex vivo study of blood antioxidant status after food consumption. The bioavailability of dietary phytochemicals as well as the metabolism of food antioxidants, i.e., the potential transformations of phytochemicals through gut microbiota and intestinal and hepatic metabolism, may be taken into account when assessing serum/plasma AC after food intake 71. This may provide a more comprehensive picture of the true impact of food antioxidant assumptions on blood antioxidant status, which goes beyond the AC of the consumed food and cannot be determined solely by in vitro AC measurements.
Unfortunately, this strategy may have some flaws, leading to some contentious outcomes. In fact, blood AC measurements after ingestion of antioxidant-rich foods have shown contrasting results, with only a small increase 72,73, no effect 74,75, and in some cases, even a decrease 35,75. It's also worth noting that some studies found no improvement in AC followed by a decrease in serum/plasma oxidation levels 35,76. These results indicate that blood AC tests alone are unsuitable for determining food antioxidant efficacy, and that changes in serum oxidative status must also be considered. In light of this, an improvement in the calculation of serum antioxidant status was attempted by proposing a novel parameter called "Antioxidant/Oxidant Balance" as a means of overcoming this flaw (AOB). It is obtained as a ratio between serum AC and serum oxidant status, evaluated as "Peroxide Level" AOB = AC/Peroxide Level (PxL) 34. It includes evaluation of both antioxidant and oxidant status of blood after food intake and is obtained as a ratio between serum AC and serum oxidant status, evaluated as "Peroxide Level" AOB = AC/Peroxide Level (PxL) 34. It should be noted that the results of PxL's serum hydroperoxides assay 34 are strongly associated with those of the oxidised low-density lipoproteins assay 34.
For the first time, the efficacy of the novel AOB method in illustrating improvements in serum antioxidant status after food intake was evaluated by comparing the short-term (up to 4 h) effects of two antioxidant-enriched pastas, supplemented with bran oleoresin (BO) and bran water (BW) extracts, respectively, to a non-supplemented reference (R) pasta 34. Initially, the efficiency of the AOB parameter was verified following the consumption of two foods that are supposed to have the opposite effect on serum AC. They included Lisosan G, a dietary supplement with a high in vitro AC of 26,30 (see also Table 3) and glucose, which is considered to have a pro-oxidant effect 77,78. AOB profiles obtained within 4 hours after Lisosan G and glucose assumption by measuring ACLOX–FL/PxL, ACORAC/PxL, or ACTEAC/PxL ratios are compared to AC and PxL profiles in Laus et al. 34. Although serum AC measurements varied between assays 79, AOB profiles measured as ACLOX–FL/PxL, ACORAC/PxL, or ACTEAC/PxL all agreed on a significant improvement in serum antioxidant status after Lisosan G intake, as well as a significant decrease due to glucose ingestion 34. In the case of pastas, all AOB profiles showed a decrease in serum antioxidant status after R pasta consumption, comparable to glucose; a decreasing trend was also seen after R pasta consumption combined with Lisosan G 34. In a similar way to Lisosan G 34, BO pasta intake resulted in a substantial increase in AOBLOX–FL, as well as AOBORAC or AOBTEAC. BW pasta, on the other hand, was not only unable to have a beneficial effect on serum, but it also had an oxidative effect, as did R pasta and glucose.
Q16) What is Hoffmann’s exhaustive methylation?
A16)
Exhaustive methylation is the process of alkylation with methyl groups that continues until all possible methylations are achieved.
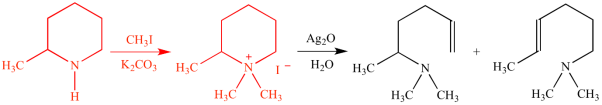
Prior to E2 elimination, exhaustive methylation (shown in red) transforms an amine to an alkylammonium salt in the Hofmann elimination reaction chain.
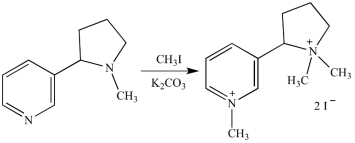
In natural product structural analysis, exhaustive methylation has been used. If one mole of an unknown alkaloid (in this case, nicotine) reacts with two moles of methyl iodide, we can deduce that the alkaloid has two methylation sites.
Q17) What is Hoffmann Elimination?
A17)
Hofmann eliminations are 4o-ammonium salt reduction reactions. Since the counter anion in most 4o-ammonium salts is halide, it is frequently replaced by the more simple hydroxide ion via silver hydroxide reaction (or silver oxide). To impact the E2-like removal of a 3o-amine, the resulting hydroxide salt must be heated (100 - 200 oC). A typical Hofmann elimination is shown in Example #1 below. As previously mentioned, when looking at alkyl halide elimination reactions, one of the alkyl substituents on nitrogen must have one or more beta-hydrogens for an elimination to occur.
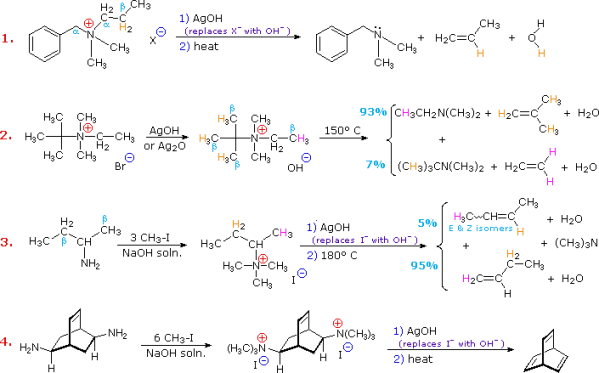
Two of the alkyl substituents on nitrogen in Example #2 have beta-hydrogens, all of which are on methyl groups (colored orange & magenta). The alkene with the more strongly substituted double bond is the main product of the removal, reflecting not only the 3:1 numerical advantage of certain beta-hydrogens, but also the greater stability of the double bond. Example #3 highlights two main aspects of the Hofmann elimination:
1. Extensive alkylation, typically with methyl iodide, converts simple amines to the necessary 4o-ammonium salts (methyl has no beta-hydrogens and cannot compete in the elimination reaction). In example #4, exhaustive methylation is demonstrated once more.
2. When an alkyl group has two sets of beta-hydrogens available for removal (coloured orange and magenta here), the alkene isomer with the less substituted double bond is always the main product.
The Hofmann Rule refers to the tendency of Hofmann eliminations to produce the less-substituted double bond isomer, which contrasts sharply with the Zaitsev Rule for dehydrohalogenations and dehydrations. The Hofmann Rule does not apply when other triggering groups, such as phenyl or carbonyl, are present. When 2-amino-1-phenylpropane is handled in the manner described in Example #3, the result is primarily 1-phenylpropene (E & Z-isomers).
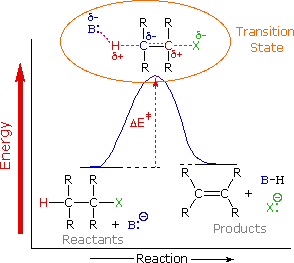
It's important to reexamine the existence of the E2 transition state, which was first identified for dehydrohalogenation, to understand why base-induced removal of 4o-ammonium salts behaves differently than that of alkyl halides. On the right, the energy diagram for a single-step bimolecular E2 process is repeated. E2 reactions have a less well-defined transition state than SN2 reactions. More bonds are breaking and forming, with the probability of a continuum of states where the degree of C–H and C–X bond breaking and C=C bond formation varies. The transition state resembles that of an E1 reaction if the bond to the leaving group (X) is significantly broken relative to the other bond changes (initial ionisation followed by a fast second step). On the other hand, if the beta-hydrogens' acidity is increased, significant C–H bond breaking can occur before the other bonds are affected. For most simple alkyl halides, it was necessary to imagine a balanced transition state in which all bonds changed at the same time. The Zaitsev Rule was compatible with such a model.
Q18) What is Interactions between nicotine and drugs of abuse?
A18)
● Introduction
In the United States, nicotine-containing products are among the most commonly used addictive drugs. According to the most recent Substance Abuse and Mental Health Services Administration (SAMHSA) survey, 67 million people (nearly 25% of the population) use tobacco. Despite the fact that the number of people who use tobacco products has declined over the last decade, the use of modern products like nicotine-containing e-cigarettes and other electronic nicotine delivery systems (ENDS) has skyrocketed. In addition to the serious problem of nicotine addiction in the general population, cigarette smoking among people who have substance use disorders is a specific public health issue that has received insufficient attention, particularly in preclinical research. Since nicotine is a legally available drug with relatively mild psychoactive effects, the full extent of nicotine polysubstance usage is likely underreported. Even so, nicotine is frequently mentioned as a portion of the most common polysubstance combinations. In a European study of school students, nearly three-quarters (73%) of polysubstance users reported using both alcohol and cigarettes in the previous month, and one-fifth of those surveyed reported using cannabis (9-tetrahydrocannabinol; THC) concurrently with alcohol and/or nicotine.
The societal consequences of nicotine polysubstance usage are a significant source of concern. Chronic health issues linked to nicotine addiction are one of the leading causes of preventable death in the United States (e.g., 480,000 deaths per year). Tobacco use results in annual economic costs of $289 billion for cancer, cardiovascular disease, and other health risks. Furthermore, the health risks associated with medication combinations greatly outweigh the risks associated with any drug alone. As a result, polysubstance consumers often report, and laboratory tests confirm, that mixing multiple substances enhances the use of one or both of them (e.g., cocaine, d-amphetamine, heroin, and alcohol). This increases the urgency of the problem and the need for strategies to control and/or counteract the effects of this type of polysubstance abuse. This can lead to accelerated development of dependency or increased incidences of acute or long-term toxicity – reduced liver or kidney function, damage to the cardiovascular or respiratory systems, etc.– and emphasises the urgency of the problem and the need for strategies to manage and/or counteract the effects of this type of polysubstance abuse.
The interactions of nicotine with alcohol, stimulants (e.g., cocaine, amphetamines), opioids (e.g., morphine, heroin, etc.) and THC are the subject of this study. Work in some of these areas is limited, as should be obvious. The studies discussed below will illustrate the current understanding of how these various groups of abused drugs could interact with nicotine on behavioural, physiological, and pharmacological indices that may be important in maintaining co-use of either or both substances, while not intended to be exhaustive. There are some ideas for potential research areas and knowledge gaps discussed.
It is widely acknowledged that nearly 80% of nicotine-dependent adult smokers began smoking during their teenage years. Furthermore, teens who smoke cigarettes are seven times more likely than those who do not to use an illegal drug. Similarly, sex/gender differences can play a role in determining behavioural and/or neurochemical responses to drugs of abuse, as well as drug use patterns. A number of recent reviews have looked at the effects of nicotine/tobacco or other substance use during crucial developmental phases (prenatal or adolescent), as well as the effects of sex/gender on behavioural and neurobiological endpoints of drug exposure. As a result, a thorough examination of the literature is beyond the reach of this study.
Q19) What is Terpenes?
A19)
Two or more isoprene molecules are connected together in the biosynthesis of terpenes. Provided that the head and tail of the molecule are mainly involved in the linking, there are three possibilities for linking between two isoprene molecules:
Linkage
One isoprene molecule's head may connect to the head of another isoprene molecule.

Figure: This link is called a head-to-head or 1-1 link.
Linkage
One isoprene molecule's head may connect to the tail of another isoprene molecule.

Figure: This link is called a head-to-tail or 1-4 link.
Linkage
One isoprene molecule's tail may connect to the tail of another isoprene molecule.

Figure: This link is called a tail-to-tail or 4-4 link.
Crosslinks are found in cyclic terpenes that are not 1-1, 1-4, or 4-4.
Eg. 1:
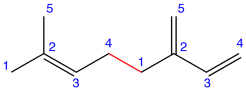
Figure: Myrcene
Eg. 2:
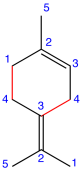
Figure: Limonene
Eg. 3:
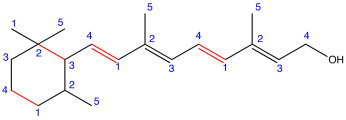
Figure: Retinol
An irregular terpene is one that does not follow the isoprene law. As an example:
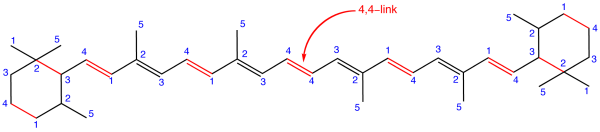
Figure: β-Carotene
Natural occurrence:
Because of their high reactivity, most metals are found in nature in a combined state. Minerals are metals found in a mixed state in the earth's crust. Ores are minerals that have a relatively high metal content and can be processed in a simple and cost-effective manner.
Gold and platinum are the only metals that can be found in their natural state. They are commonly embedded in the earth's crust with earthly (or) rocky impurities like sand and clay.
Metal abundance varies in the earth's crust.
By weight, the relative abundance of certain metals in the earth's crust is:
Metal | Relative Abundance |
Al | 8.23 |
Aluminium and iron are more plentiful than other metals. By weight, aluminium accounts for around 8% of the earth's crust, while iron accounts for 5.8%.
As a result, aluminium is the most abundant metal in the earth's crust, and iron is the second most abundant metal by weight. In addition, by weight of the earth's crust, aluminium is the third most abundant element, and iron is the fourth most abundant element.
The main ores of certain essential metals, as well as their chemical composition:
Metal | Ores | Composition |
Aluminium (Al) | Bauxite, | AlOx(OH)3-2x [Where 0<x<1] |
Iron (Fe) | Haematite | Fe2O3 |
Copper (Cu) | Copper pyrites | CuFeS2 |
Zinc (Zn) | Zinc blende or Sphalerite | ZnS |
Aluminium and iron are commonly used in industrialised countries. Aluminium is a major component of silicates like mica and clay that occur naturally. Aluminium is mostly derived from bauxite, which is the primary ore.
Aluminium oxides, as well as traces of transition metals, are found in valuable gem stones like rubies and sapphires. Rubies are crystalline types of aluminium oxide that are reddish-brown in colour due to chromium impurities. Due to the presence of impurities such as nickel, cobalt, and titanium, sapphires come in a variety of colours.
Haematite and magnetite are the most common oxide ores from which iron is extracted. The iron extracted from these ores is primarily used to make steel and iron alloys. Iron is also a necessary component of biological systems.
Copper and zinc are two other metals that are widely used.
Q20) Give the Elements of modern periodic table.
A20)
Five elements make up the group 16 elements of the current periodic table: oxygen, sulphur, selenium, tellurium, and polonium. Since several elements can be extracted from sulphide or oxide ores, these elements are also known as chalcogens or ore-forming elements.
On the surface of the earth, oxygen is available. After hydrogen, helium, and neon, oxygen was believed to be the fourth most abundant element in the universe, based on the proportions of various forms of atoms present in the universe. It makes up 89 percent of water, 46 percent of the earth's crust, and 20% of the atmosphere.
Period | Element | Symbol | Atomic Number | Electronic Configuration |
2 | Oxygen | O | 8 | [He] 2s2 2p4 |
3 | Sulphur | S | 16 | [Ne] 3s2 3p4 |
4 | Selenium | Se | 34 | [Ar] 3d10 4s2 4p4 |
5 | Tellurium | Te | 52 | [Kr] 4d10 5s2 5p4 |
6 | Polonium | Po | 84 | [Xe]4f14 5d10 6s2 6p4 |
Oxygen:
Oxygen is represented by the chemical symbol O. It is a colourless, odourless gas that is converted into carbon dioxide during the human respiration process. The molecule of oxygen is a diatomic molecule (O2). In traces, oxygen can also be present as a triatomic molecule (O3), which is known as ozone. Many elements mix easily with oxygen. The evolution of heat energy occurs during the combination of certain components, and this process is known as combustion.
Sulphur:
Sulphur is represented by the letter S. It is a non-metal that comes in ninth position in terms of celestial abundance. Sulphur atoms make up about one in every 20,000-30,000 atoms. Sulphur can be present in both the combined and free states. Seawater contains around 0.09 percent sulphur in the form of sulphates. A considerable volume of sulphur is contained in underground deposits of pure sulphur present in dome-like structures, and the meteorite contains 12 percent sulphur. The activity of anaerobic bacteria on sulphate minerals such as gypsum produces sulphur.
Selenium:
Selenium is more scarce than either oxygen or sulphur. In a few minerals, it can be found both free and mixed with heavy metals (such as iron, silver, or mercury). Under normal conditions, the grey metallic form of selenium is the most stable form of the element.
Tellurium:
Tellurium is a chemical element with the atomic number 52 that has properties that are similar to both metals and non-metals. It's one of the rarest stable elements on the planet's surface. It's commonly found in free form and in compounds with other elements including copper, lead, silver, and gold.
Polonium:
Among the group 16 elements, it is the rarest. It's a radioactive substance. Polonium is also used for alpha radiation in research applications.