Unit - 1
Organometallic Compounds-I
Q1) Explain the Classification of Organometallic Compounds?
A1)
(i) Sigma bonded organometallic compounds: For example, in Grignard reagents, R – Mg – X, where R is an alkyl or aryl group and X is a halogen, the metal atom and the carbon atom of the ligand are bonded together with a sigma bond.
(b) Zinc compounds of the formula R2Zn, such as (C2H5)2Zn, (C2H5)2Zn, (C2H5)2Zn, (C2H5)2Zn, (C2H5)2Zn, (C2H5)2Zn, (C2H5)2Zn, (C2H (isolated by Frankland).
(ii) Pi-bonded organometallic compounds: Metal compounds with alkenes, alkynes, benzene, and other ring compounds are known as alkenes, alkynes, benzene, and other ring compounds. The metal and ligand establish a connection in these complexes that involves the ligand's -electrons. Zeise's salt, ferrocene, and dibenzene chromium are three common examples.
(iii) Sigma and Pi bonded organometallic compounds: This class includes metal carbonyl compounds, which are generated when metal reacts with carbon monoxide. Both - and -bonding are present in these compounds. In these compounds, the oxidation state of metal atoms is usually zero
Q2) What is hapticity of an organometallic ligand?
A2)
The hapticity of an organometallic ligand is a straightforward concept: it simply indicates how many atoms in the ligand are linked to the metal centre. The Greek letter “eta” is commonly used in chemistry literature to represent this. A ligand with two atoms bound to the metal centre is called a 2, a ligand with one atom is called a 1, and so on. The most frequent hapticity for a ligand is 1, though ligands with higher coordination numbers, such as the cyclopentadienyl anion ligand (typically 5) are not uncommon.
When a lone pair is bonded to a transition metal, the lone pair's atom forms a bond with the metal. Which atom is connected to the metal when a pi bond is formed? They're both of them. This bonding condition involves three atoms rather than just two. Both carbon atoms that make up the original pi bond are now donating it to the metal.
Q3) Why Cl – ion trans to the ethylene has the large Pt –Cl bond distance than the other two Pt –Cl bond distance in Zeise’s salt?
A3)
The trans orienting power of ligands can be used to explain the above finding. Due to back bonding, the metal olefin bond has multiple bond character, and this multiple bond character (-bonding effect) reduces the metal–carbon bond length. As a result, the metal chlorine connection linked to the ethylene molecule weakens, resulting in an increase in the length of the Pt–Cl bond. However, this -bonding has no effect on the Pt–Cl bond character cis to the C2H4 molecule. As a result, the trans Pt–Cl bond distance is greater than the other two Pt–Cl bond distances.
Q4) Is the rotation of C2H4 molecule in Zeise’s salt hampering the stability of the complex?
A4)
The ethylene molecule can either lay on the same plane as the PtCl3 unit or lie perpendicular to the plane of the molecule, based on bonding in Zeise's salt.
Back bonding is conceivable in each scenario. Back bonding occurs in the first scenario via a filled dxy metal orbital, while it occurs in the second instance via dyz or dxz metal orbitals.
Because the probability of such back bonding is larger in the second situation, the stability is increased.
Q5) Why the ethylene molecule is perpendicular to the PtCl3 molecular plane in Zeise’s salt?
A5)
In Zeise’s salt the metal ion, Pt (II) contains three π-type filled d-orbital which are dxy, dyz, and dxz.
When the coordinated ethylene molecule lies perpendicular to the molecular plane, the back bonding may take place either through dyz or dxz filled orbitals, but it may take place only through dxy filled orbital. When C2H4 molecule exists in the plane of the molecule. As a consequence, C2H4 molecule lies perpendicular to the PtCl3 molecular plane for better scope of back bonding.
Q6) Why the C –C olefinic bond length in Zeise’s salt is greater than the C –C bond in free hydrocarbon?
A6)
Back bonding from a suitable full metal d orbital into the unoccupied anti bonding*orbital on the carbon atom occurs in Zeise's salt. The C–C bond order should be decreasing in the unbound C2H4 molecule.
As a result, the C–C bond length in Zeise's salt increases from 1.34 for a free C2H4 molecule to 1.4-1.47 for a coordinated C2H4 molecule
Q7) What is Zeise’s salt? Discuss the structure and bonding of Zeise’s salt?
A7)
Platinum (Pt), a 4d transition metal, which forms a large number of organo-metallic compounds. Zeise’s salt is one of them.
The salts containing the ion [ C2H4PtCl3] -1 are known as Zeise’s salt after the name of discover such salts may be K[C2H4PtCl3], Na[C2H4PtCl3], NH4[C2H4PtCl3] etc.
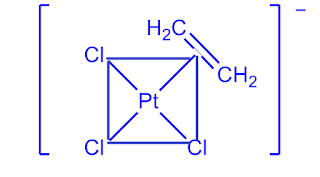
Fig: Structure of Zeise’s Salt
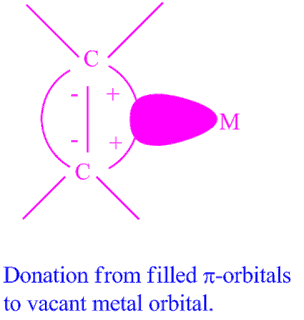
The molecular orbital (M.O) technique can explain the bonding in Zeise's salt. The metal-to-olefin bond is thought to be made up of two pieces, according to the notion.
(I) Overlap of the olefin's -electron density with a metal atom's -type acceptor orbital.
(II)Back bonding caused by electron density flow from a filled metal d orbital into antibonding unoccupied C-atom orbitals.
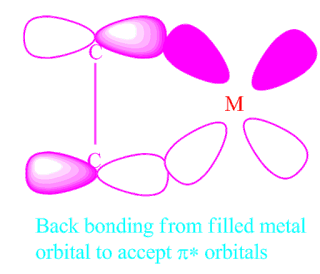
This perspective is related to carbonyl bonding, but differs somewhat, and is referred to as -bonding.
Q8) Explain the Preparation of Zeise’s salt?
A8)
When platinum tetra chloride reacts with ethanol, it forms an adduct, which when combined with KCl forms Zeise's salt. The two methods for making Zeise's salt are shown here.
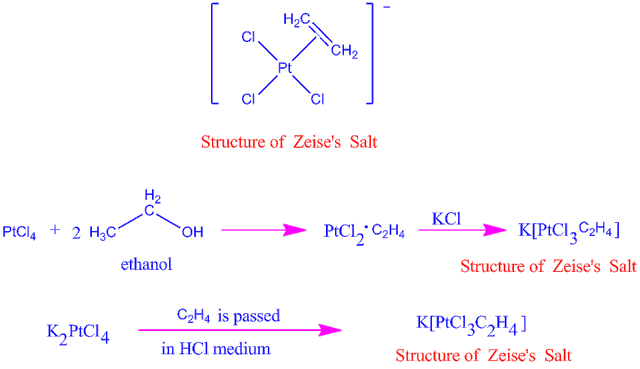
The nature of bonding in an olefinic compound like Zeise's salt is still unknown, although it is obvious that there are no localized -bonds between metal atoms and carbon.
The interaction between the -electrons in the unsaturated molecule and the metal atom's hybrid orbitals is commonly attributed to this. The X-ray diffraction method has been used to investigate several olefin compounds.
Q9) Explain the Syntheses of metal carbonyls?
A9)
Metal carbonyls can be created in a number of different methods. Here are a couple such examples: Direct interaction with the metal can produce homoleptic or binary metal carbonyls for Ni and Fe (Equation 1). In other circumstances, a metal precursor is reduced in the presence of CO (or with CO as the reductant) (Equations 2-3). Carbon monoxide interacts with a variety of metal complexes, most commonly occupying a vacant coordination site (Equation 4) or performing ligand substitution events (Equation 5). (Equation 5). CO ligands are occasionally produced through a deinsertion event involving a coordinated ligand (Equation 6).
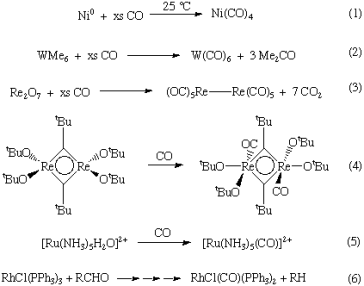
Q10) How to rationalize with MO theory that CO is a two-electron donor through carbon?
A10)
The main distinction between these molecules is that CO is heteronuclear, which means that the energy levels of the molecular orbitals and the atoms will differ.
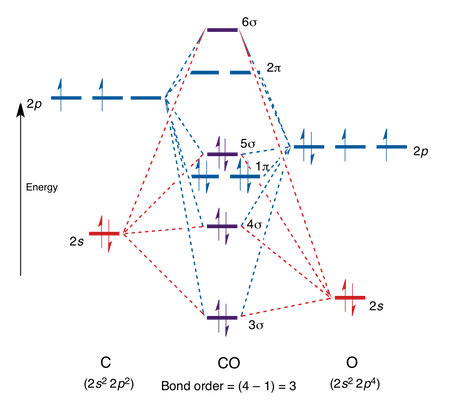
The HO is represented by the number 5 in the MO diagram above.
Q11) Explain the Olefin complexes?
A11)
The oldest known organometallic compound is Zeise's salt, K[PtCl3(C2H4)], which was produced and studied by Zeise about 1825, however its coordination structure was only hypothesized in 1954 and confirmed by neutron diffraction in 1975. The Dewar-Chatt-Duncanson model describes the manner of coordination of an olefin to a transition metal, and the bond between the metal and the olefin is stabilized by the contribution of d-p back donation. An olefin is a two-electron ligand, and many olefin complexes have a core metal that is in a low oxidation state. Dienes or trienes with two or more double bonds act as 4-electron or 6-electron ligands to bind to metals. Examples are Fe (CO)3(C4H6) and Ni(cod)2, in which a butadiene or cyclooctadienes (cod) are coordinated to the metal. Because cyclooctadienes are easily removed from Ni(cod)2, it's an easy way to get atomic, zero-valent nickel. This compound is also known as "bare nickel."
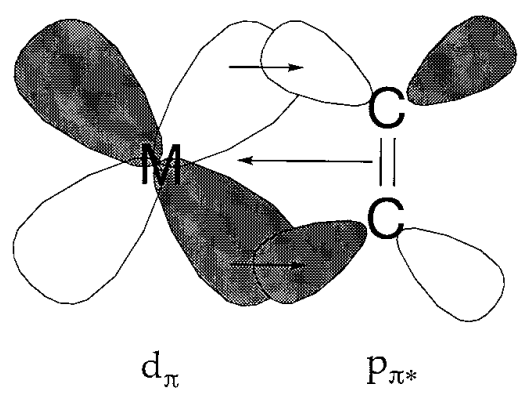
Q12) Explain the Electron Counting covalent Methods?
A12)
All metal-ligand bonds are deemed covalent in this manner. Ligands have no charge and can give either two, one, or zero electrons to the bond. For instance, ligands with a filled valence, such as CO and NH3, contribute two electrons. In their neutral state, halide and hydroxo groups, on the other hand, do not have an octet structure and contribute one electron to bonding. Ligands like BF3 have no free electrons, thus the two electrons needed for bonding must come from the metal centre.
The steps for using the covalent counting method are as follows:
1. Determine the metal center's group number.
2. Determine how many electrons the ligands contribute.
3. Determine the metal-ligand complex's total charge.
4. When a metal-metal bond exists, one electron is assigned to each metal centre in the bond.
5. To get the final electron count, add the group number of the metal centre and the e- count of the ligands, then factor in the overall charge of the complex.
Q13) Explain the Electron Counting Ionic Method?
A13)
The ligands are always assigned filled valences in the ionic technique. For example, the H group, as well as other groups like halide, hydroxyl, and methyl, are now termed H-. These groups now contribute one more electron than in the covalent approach, and when a bond is formed, they oxidize the metal core. CO and NH3, for example, have a neutral charge in their octet structure and react similarly to valence techniques.
The following are the steps in the ionic counting method:
1. Determine the metal complex's total charge.
2. Determine the ligands' charges and the quantity of e-s they give.
3. Determine the amount of valence electrons in the metal centre so that the overall charge of the complex is balanced by the metal's oxidation state and the charges of the ligands. (E-count of metal centre = Metal atom group number + (charges of ionic ligands) – complex overall charge)
4. If a metal-metal bond exists, one bond equals one electron per metal atom.
5. Add the metal centers and ligands' electron counts together.
Q14) What is general rule and exception to the rule?
A14)
The General Rule
All of an atom's valence orbitals are usually occupied by paired electrons. The valence orbitals of transition metals are composed of ns, 3 np, and 5 (n-1) d orbitals, resulting in a tendency to be surrounded by 18 electrons. In a simplified explanation, this is similar to the octet and Lewis structural principles of major group elements.
Electron-precise structures are those that satisfy this chosen electron structure. There will be no more unoccupied low-lying orbitals accessible for further ligand coordination in transition metal complexes with 18 electrons, which are also known as saturated. Unsaturated complexes have less than 18 electron counts and can electronically bind to additional ligands.
Exceptions to The Rule
In metal complexes with strong field ligands that are good donors and acceptors, the 18-electron rule is frequently followed (for example, CO ligands). Because the energy difference (0) between the t2g and eg* orbitals is so enormous, the three t2g orbitals become bonding and are always full, whereas the two eg* orbitals are strongly antibonding and are always empty in this situation.
Q15) Explain the Metal carbonyl compounds?
A15)
Binary metal carbonyl compounds containing only a metal and CO ligands are commonly made by reacting a highly reactive metal powder with carbon monoxide directly, or by reducing a metal salt to zero valence and then reacting with high-pressure carbon monoxide. Tetracarbonylnickel, on the other hand, is formed by the interaction of nickel metal with carbon monoxide at room temperature and atmospheric pressure. It was originally found near the end of the nineteenth century. Other metal carbonyl compounds, on the other hand, necessitate high temperatures and pressures for synthesis.
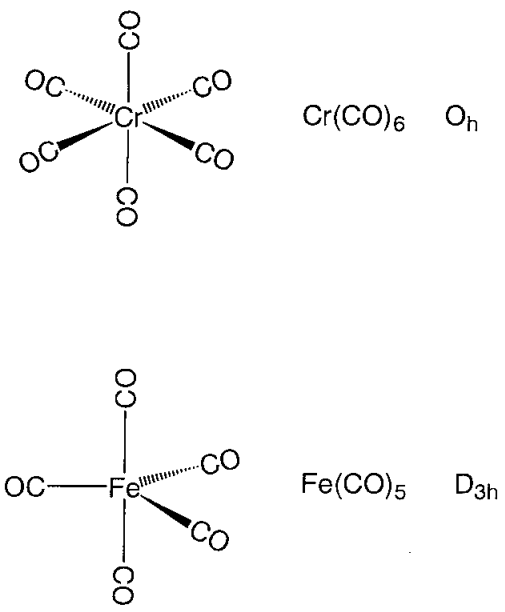
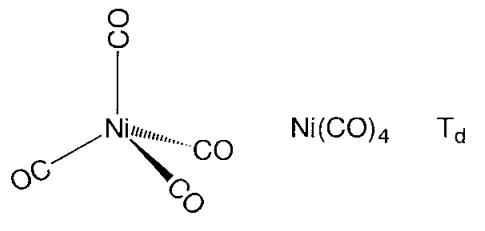
Figure- Structures of metal carbonyl compounds.
The coordination structures of mononuclear metal carbonyl compounds are highly symmetric polyhedral. Hexa-coordinate chromium, molybdenum, and tungsten hexacarbonyl, M(CO)6, has a regular octahedral coordination structure, while penta-coordinate pentacarbonyliron, Fe (CO)5, has a triangular bipyramid coordination structure, and tetracarbonylnickel, Ni (CO)4, has a regular tetrahedron coordination structure
Q16) Explain the Alkyl ligands?
A16) M-C single bonds are found in alkyl or aryl transition metal complexes. Despite numerous attempts over the course of chemical history, they were never successfully isolated, and it was long assumed that all M-C bonds were inherently unstable. Only in the 1950s did stable alkyl complexes begin to be created. Some representative compounds include Cp2ZrCl(Pr), WMe6, CpFeMe(CO)2, CoMe(py)(dmg)2, IrCl(X)(Et)(CO)(PPh3)2, NiEt2(bipy), and PtCl(Et)(PEt3)2. The reactions of compounds having M-halogen bonds with main-group metal-alkyl compounds, such as a Grignard reagent or an organolithium compound, are frequent synthetic routes among the numerous synthetic procedures so far established. Vitamin B12, whose structure was established by D. Hodgkin (1964 Nobel Prize), is known to have a particularly stable Co-C bond. Homoleptic alkyls, such as WMe6, are metal alkyl compounds with only an alkyl ligand.
Q17) Explain the π allyl complexes?
A17)
An allyl group, CH2=CH-CH2-, is a 1-electron ligand like an alkyl group when it is connected to a metal via a carbon atom. Three carbon atoms link to the metal concurrently as a 3-electron ligand if the double bond delocalizes. This is an odd electron ligand that is nominally anionic and is stabilized by being coordinated to the metal.
Examples include Pd(C3H5) (Ac)(PPh3), Co(C3H5)3, and others. Various reactions occur in the catalytic reactions of unsaturated hydrocarbons because coordination modes of 11, 22, and 33 are feasible.
C5H5 is the abbreviation for the cyclopentadie nyl ligand. C5Me5, a beneficial ligand dubbed Cp star and denoted by Cp*, is a helpful ligand in which the hydrogen atoms of Cp are substituted by methyl groups. Ferrocene (Cp2Fe) is an orange-colored iron compound with two cyclopentadienyl groups attached to it.
Q18) What are the Phosphine complexes?
A18)
PX3 tertiary phosphines are excellent stabilizing ligands for transition metal complexes, and they coordinate to metals in a wide range of oxidation states. In the chemistry of organometallic complexes, phosphones are widely utilized as carbonyl or cyclopentadienyl ligands. PX3 are Lewis bases that coordinate to metals utilizing the lone pair on phosphorus and exhibit -acidity when substituents X, such as Ph, Cl, or F, have significant electron accepting characteristics. PX3's electrical versatility is what allows it to generate so many compounds. In general, the -acidity decreases in the following order: PF3 > PCl3 > PPh3 > PR3. Typical substituted phosphines are triphenylphosphine and triethylphosphine. Table 6.4.76.4.7 lists the tertiaryphosphine complexes, which are mostly metal halide complexes. Only a few phosphine complexes are formed by manganese, Mn, and the early transition metals.
Q19) Explain the Electron configuration of high and low spin?
A19)
The SALCs of the ligands are occupied in a -donor ligand, therefore it transfers electrons to the molecular * and * orbitals. Because the orbitals linked with e.g., are not involved in interactions, it maintains its energy level (Figure 1.11.1). The occupied ligand SALC t2g orbitals (i.e., dxy, dxz, dyz) that would form molecular orbitals with the metal t2g orbitals (i.e., dxy, dxz, dyz) have a lower energy than their metal counterparts. The resulting MO has * orbitals that are less energetic than the * orbitals generated by the non-bonding orbitals (eg)., split denotes the difference between the t2g* and eg orbitals. The is tiny in the -donor instance due to the low * level.
Because the t2g SALCs of pi accepting orbitals are vacant, they have a higher energy than metal t2g orbitals. The t2g* orbitals that result is higher than the * orbitals. This results in a wider gap between the eg and t2g orbitals, resulting in high split ligands for these -accepting orbitals.
Finally, the size of as determined by the ligand's identity will determine how electrons are dispersed in metal d orbitals (Figure 1.11.2). Weak field ligands cause a small spin configuration, resulting in a high spin configuration. Strong field ligands induce a large spin configuration on the d electrons, resulting in a low spin configuration.
Q20) Explain the Spectroscopic Features of Carbonyl Complexes?
A20)
The IR spectra of carbonyl compounds show two useful features that are both consistent with the concept of pi-back bonding mentioned above:
The CO stretching frequency drops by around 100 cm-1 with each charge applied to the metal centre
The lower the CO stretching frequency, the better the sigma-donating capability (or the worse the pi-acceptor capability) of the other ligands on the metal.
Counting the number of IR and Raman CO stretching frequencies allows one to make a structural assignment for simple carbonyl compounds. Group theory can be used to predict the number of CO stretches expected for certain geometries/isomers, and the computed findings can then be compared to experimental data.
Coordinated carbonyl ligands often appear in the range of 180 to 250 ppm in the 13C NMR spectra. Carbonyl compounds with isotopically enriched isotopes are frequently synthesised to simplify mechanistic research or to make spectrum gathering easier. Furthermore, connecting a 13C-enriched compound to additional spin active nuclei like 103Rh or 31P can help with structural assignments.