Unit - 2
Enzymes
Q1) What are Enzymes? Enlist their Fundamental Characteristics? Explain the Classification of Enzymes?
A1) Enzymes are molecules They're unique proteins. Enzymes are quite good at breaking certain bonds and replacing them with new ones in these domestic applications. They're breaking down larger molecules in the process, which could result in a stain or a delayed drain. Those larger molecules break down into smaller molecules that are easier to wash away.
The most fundamental characteristics are:
• Proteins are made up of chiral amino acids
• Enzymes are proteins
• Enzymes are large, chiral molecules
Classification Of Enzymes:
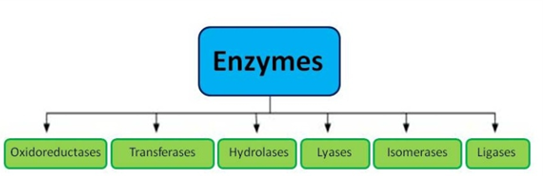
- Oxireductase
By withdrawing or adding electrons from or to their substrates, these enzymes catalyse oxidation or reduction. Dehydrogenase, oxidase, and reductase are examples of enzymes.
- Transferase
These enzymes move a group of molecules from one substrate to another. Transaminase, for example.
- Hydrolases
These enzymes break certain covalent bonds and hydrolyze water to break bigger molecules into smaller ones. Carbohydrase, for example.
AB + HOH -> AH+BOH (AB Substrate)
- Lyases
Without hydrolysis, these enzymes catalyse the breaking of particular covalent bonds and the elimination of groups. Histidine decarboxylase, for example.
- Isomerases
Isomerases are enzymes that catalyse the rearranging of atoms inside a molecule to generate isomers. Phosphohexoisomerase, for example.
- Ligases
Ligases catalyze the formation of C - C, C - S, C - O and C - N bonds. The energy for the reaction is derived by the hydrolysis of ATP. Eg. Pyruvate carboxylase.
Q2) Describe the Properties of Enzymes.
A2)
1. The enzymes, like inorganic catalysts, are active in extremely little concentrations and stay unaltered when the reaction is completed.
2. Enzymes have a fairly narrow range of action. i.e., a specific enzyme catalyses a specific sort of reaction by acting on a specific substrate. However, the same process may be performed by many enzymes at times. Isoenzymes are enzymes that work in this way.
3. Enzymes are extremely heat sensitive. The temperature at which an enzyme is most active is referred to as its optimal temperature. Both above and below the optimal temperature, enzyme activity decreases. The catalytic component of the enzyme is made up of proteins. When the temperature rises beyond 50°C, the protein denatures, and the enzyme loses its action.
4. The enzyme's catalytic property is affected by its pH. The optimal pH is the pH at which the enzyme activity is highest. The optimal pH value for various enzymes is variable.
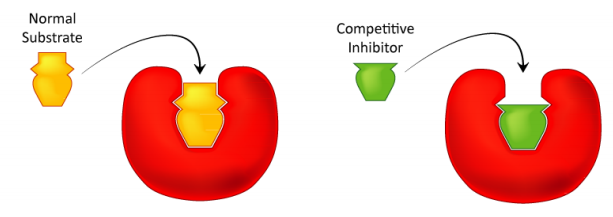
5. Inhibitors have a strong effect on enzymes. An inhibitor is a chemical compound that binds to enzymes and prevents them from catalysing reactions. Cyanides, for example.
A-><-B.
The reactions catalysed by enzymes are usually reversible, depending on the needs of the cell.
• Although a few catalytically active RNA molecules have been discovered, nearly all enzymes are proteins.
• When contrasted to chemical reactions, enzyme catalysed reactions normally take place under comparatively mild conditions (temperatures considerably below 100oC, air pressure, and neutral pH).
• Enzymes are catalysts that speed up the rate of a chemical reaction without changing the reaction itself.
• Enzymes are quite particular in terms of the substrates they operate on and the products they produce.
• Enzyme activity can be controlled, fluctuating in reaction to substrate or other molecule concentrations.
• They work in the body under stringent temperature and pH parameters.
Q3) Explain the Environmental effects on enzyme function.
A3) Active sites are extremely sensitive to changes in the enzyme's environment because they are finely tailored to aid a chemical reaction. The active site and enzyme function may be affected by the following factors:
• The temperature of the room. Higher temperatures, whether enzyme-catalyzed or not, result in faster reaction rates. Increased or decreased temperature outside of a safe range, on the other hand, can disrupt chemical bonds in the active site, rendering them less well-suited to bind substrates. Very high temperatures (for animal enzymes, above 40 degrees, may cause an enzyme to denature, losing its shape and activity.
• The ph level. Enzyme function can also be affected by pH. The acidic or basic characteristics of active site amino acid residues are critical for catalysis. PH changes can alter these residues, making it difficult for substrates to bind. Enzymes perform well within a specified pH range, and excessive pH values (acidic or basic) can cause enzymes to denature, just like temperature.
Q4) Explain the Mechanism of enzyme action (Mechanism of Trypsin).
A4) Mechanism of Trypsin
Because the active sites of trypsin and chymotrypsin are identical, the mechanisms of both are identical. This process consists of four steps:
- Step 1
To begin, the hydrogen on serine's OH is removed, resulting in a negative charge on the oxygen. The extra electrons can target the peptide bond, causing the negative charge to assault it:
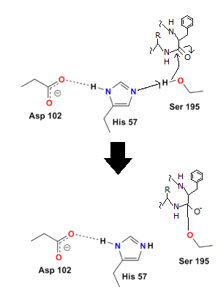
- Step 2
The oxygen's electrons can easily repair a carbon-oxygen double bond, but then another carbon bond must be formed. In this situation, the peptide's carbon-nitrogen link is broken. The histidine nitrogen provides a hydrogen to the nitrogen. The first protein residue from the enzyme has now been released:
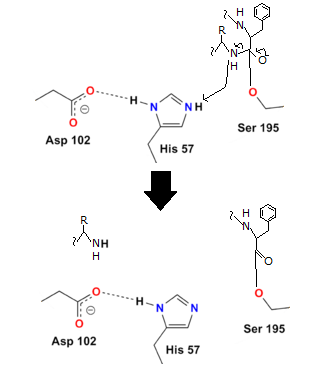
- Step 3
Although the peptide bond has been broken, the reaction has not been completed since the other half of the protein (the part containing the basic amino acid) remains linked to the enzyme. The final two steps assist the enzyme in releasing the second half of the protein.
Water is added in order to do this. The hydrogen from the water binds to the histidine, while the OH binds to the carboxyl carbon, preventing oxygen from taking on a negative charge:
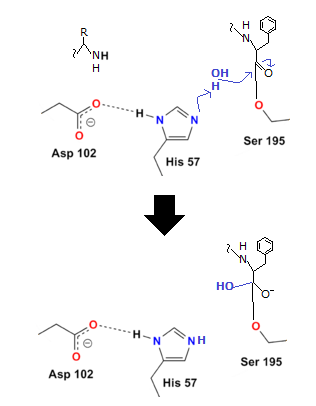
Q5) What are the Factors that affect enzyme action?
A5) Factor 1: Concentration of Enzyme
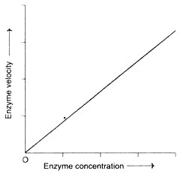
Concentration of enzymes
• As the enzyme concentration rises, the reaction velocity rises in lockstep. This feature is utilized in the diagnosis of diseases to determine the activity of serum enzymes.
Factor 2: Concentration of Substrate
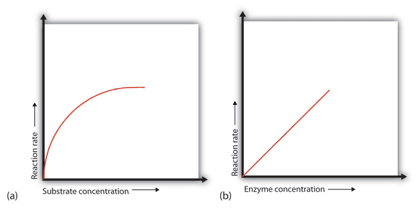
Concentration of Substrate Enzyme
• When a certain amount of enzyme is present, the rate of enzymatic reaction increases as the substrate concentration increases until a limiting rate is achieved, after which additional increases in the substrate concentration have no effect on the reaction rate. Because there is so much substrate at this phase, almost all of the enzyme active sites have substrate bound to them. In other words, the substrate has saturated the enzyme molecules.
Factor 3: Effect of Temperature
• Because enzymes are proteins, they are particularly sensitive to temperature variations. When compared to regular chemical reactions, enzyme activity occurs across a small temperature range. As you can see, each enzyme is more active at a particular temperature.
• As the temperature rises over the ideal temperature, the enzyme activity gradually decreases until it reaches a temperature at which the enzyme activity totally ceases due to a change in its natural composition.
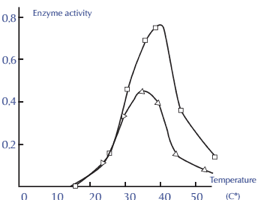
Factor 4: Effect of pH
• The best way to determine the concentration of hydrogen ion (H+) in a solution is to use the potential of hydrogen (pH). It can also tell if a liquid is acidic, basic, or neutral. Acids are liquids with a pH less than 7, whereas bases or alkalines are liquids with a pH more than 7. At 25 degrees Celsius, pH 7 liquids are neutral and have the same acidity as pure water. The pH indicators can be used to determine the pH of any solution.
Factor 5: Effect of Activators
- Some of the enzymes require certain inorganic metallic cations, like Mg2+, Mn2+, Zn2+, Ca2+, Co2+, Cu2+, Na+, K+ etc., for their optimum activity. Rarely, anions are also needed for enzyme activity, e.g., a chloride ion (CI–) for amylase.
Q6) What are Coenzymes? Explain the function of Coenzymes?
A6) Coenzymes are substances that aid in the proper functioning of an enzyme or protein. Coenzymes are organic compounds that attach loosely to an enzyme's active site to enhance substrate recruitment. Coenzymes are nonprotein, organic aid molecules for enzymes, and many vitamins are coenzymes.
Functions of Coenzymes
Apoenzymes are enzymes that don't have a cofactor. Enzymes cannot catalyse processes adequately without coenzymes or cofactors. In fact, it's possible that the enzyme won't work at all. An organism's ability to sustain life will be hampered if reactions cannot occur at the regular catalytic rate.
An enzyme becomes a holoenzyme, or active enzyme, when it acquires a cofactor. Active enzymes convert substrates into the products that an organism need to perform key chemical and physiological processes. Coenzymes, like enzymes, can be reused and recycled multiple times without affecting the rate or efficacy of the process. They bind to a part of an enzyme's active site, allowing the catalytic reaction to proceed. The coenzyme can no longer connect to the active site when an enzyme is denatured by excessive temperature or pH.
Q7) Explain the mechanism of enzymatic action.
A7) An enzyme attracts substrates to its active site, catalyzes the chemical reaction by which products are formed, and then allows the products to dissociate (separate from the enzyme surface). The enzyme–substrate complex is the combination of an enzyme and its substrates. The complex is called a ternary complex when two substrates and one enzyme are involved; a binary complex is when one substrate and one enzyme are involved. Electrostatic and hydrophobic forces attract the substrates to the active site, which are referred to as noncovalent bonds since they are physical rather than chemical bonds.

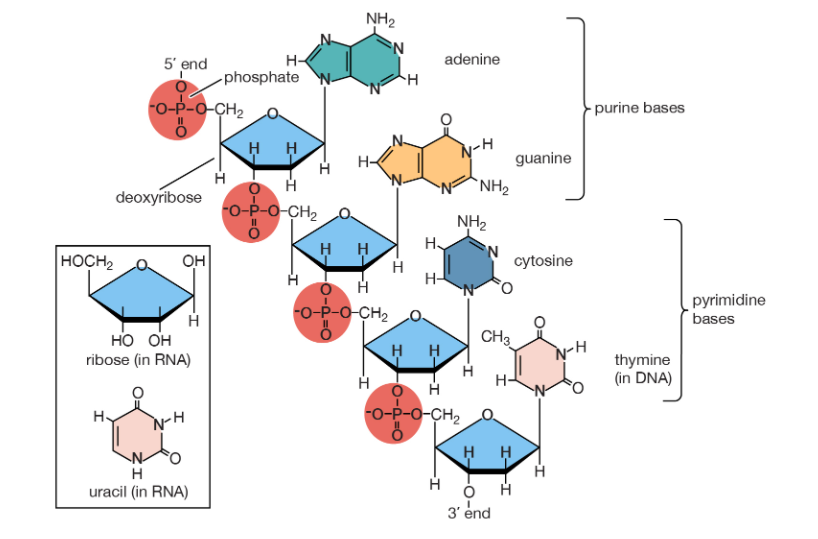
Q8) How Nucleotides Join Together?
A8) A base, a sugar, and a phosphate are all present in each nucleotide. Polymerase is an enzyme that binds the sugars to the phosphates. Each polymerase is unique to DNA and RNA.
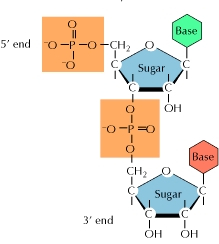
The polymerase adds additional nucleotides to the strand's bottom end, referred known as the 3' end. The phosphate group connects to the sugar's third carbon, hence the name. The phosphate group connects to the fifth carbon of the sugar at the 5' end of the strand.
One strand of a double polynucleotide, such as DNA, is upside-down with relation to the other, with its 3' end near the 5' end of the other. As a result, the two strands are known as antiparallel.
Q9) What are polynucleotide? Explain their structure.
A9) A polynucleotide is a chain of nucleotides. DNA is a pair of polynucleotides, while RNA is a polynucleotide. Nucleotides are chemical molecules with a specific makeup that make up DNA and RNA.
- Structure
Consider a row of flags, such as Tibetan prayer flags or the flags displayed at a car dealership. The string acts as a backbone, connecting the flags. A polynucleotide, like any other molecule, contains a backbone. We call this polynucleotide backbone a sugar-phosphate backbone because it is made up of the sugar and phosphate components of nucleotides.
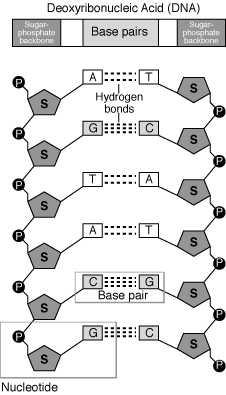
A polynucleotide strand has a sequence of bases called nitrogenous bases, just like a strand of flags can have a sequence - say, "red, red, blue, red, green..." The bases have various chemical names instead of colours. Adenine, guanine, cytosine, and thymine are the bases in DNA. This means that the sequence of a polynucleotide can look like this:
ACGTCGTATATCGTAGCTGTCAGTCGAGTAC...
RNA's polynucleotides are a little different; instead of thymine, they have uracil. They also have a different type of sugar in their backbone.
Q10) Explain the Stereochemistry of Enzyme-Catalyzed Reactions.
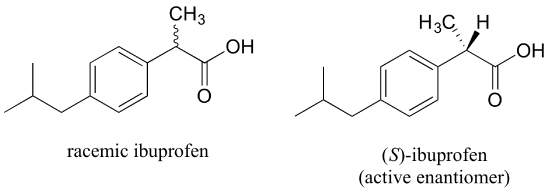
A10) Chiral centres and/or stereogenic alkene groups are found in the vast majority of biological compounds. Most significantly, proteins are chiral, which includes enzymes that catalyse a cell's chemical reactions, receptors that convey information within or between cells, and antibodies that bind selectively to potentially hazardous intruders. Proteins can recognise and bind to other organic molecules very specifically because they fold up into a certain three-dimensional structure, as you know from your biology lessons. A protein's ligand or substrate could be anything from a simple organic molecule like pyruvate to a huge biopolymer like a specific piece of DNA, RNA, or another protein. Proteins are particularly sensitive to the stereochemistry of their ligands since they are chiral molecules: for example, a protein may bind particularly to (R)-glyceraldehyde but not to (S)-glyceraldehyde, Ibuprofen is currently sold as a racemic combination, but only the S enantiomer is useful due to the specific way it binds to and inhibits the action of prostaglandin H2 synthase, an enzyme involved in the body's inflammatory response process.
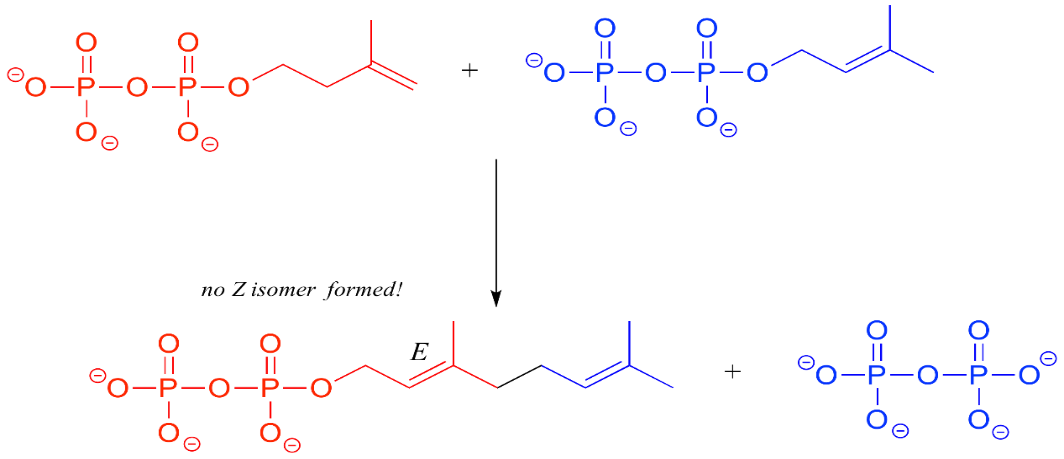
The production of the Z diastereomer is not catalysed by the enzyme.
Q11) What are Natural enzyme inhibitors?
A11) Proteins or peptides that directly bind to and inhibit target enzymes make up the majority of cellular enzyme inhibitors. These particular chemicals that are generated in organisms affect a variety of metabolic pathways. Protein serpins are an excellent example of these inhibitors. It's a huge protein family with comparable structures. The majority of them are chymotrypsin-like serine protease inhibitors.
Serine proteases (e.g., chymotrypsin) feature a reactive serine residue in their active site and comparable catalytic processes. The peptide bond is cleaved in two steps by these proteases. In the beginning of the catalytic act, a reactive serine residue in the protease active site loses H+ and becomes nucleophilic, attacking the substrate peptide bond. This causes the enzyme to release a new N-terminal section of the protein substrate (first product) and establish a covalent ester bond with the second component of the substrate. The hydrolysis of the ester bond and the release of the second product are the results of the second phase of catalysis of common substrates (C-terminal part of protein substrate.
Another example of natural inhibitors is cardiotonic steroids that were found initially in plants (digoxin, digitonin, ouabain) and in the mucus of toads (marinobufagenin, bufotoxin, etc.). These compounds are irreversible inhibitors of Na,K-ATPase that is enzyme transporting Na+ and K+ through the plasma membrane of animals against the electrochemical gradients. In the end of the twentieth century, it was shown that cardiotonic steroids are presented in low concentrations in the blood of mammals including human beings. The increase of concentration of these compounds in the blood may be involved in the development of several cardiovascular and renal diseases including volume-expanded hypertension, chronic renal failure, and congestive heart failure.
Q12) Explain Enzyme inhibitors as pharmaceutical agents.
A12) Irreversible and reversible inhibitors of prostaglandin synthesis, which affect many aspects of inflammation, smooth muscle contraction, and blood clotting, are among these medications. However, there are many additional types of medications that are enzyme inhibitors by nature; the following enzyme inhibitors are currently being developed by pharmaceutical companies and have significant therapeutic implications. Angiotensin-converting enzyme inhibitors (ACE). By removing a dipeptide from angiotensin I's C-terminus, ACE catalyses the conversion of inactive decapeptide angiotensin I into angiotensin II. The vasoconstrictor angiotensin II is very strong. The inhibition of ACE causes the concentration of angiotensin I to drop and the smooth muscles of the arteries to relax. Drugs that inhibit ACE are commonly used to treat arterial hypertension.
Inhibitors of the proton pump (PPIs). The proton pump is an enzyme found in the parietal cells of the stomach mucosa's plasma membrane. It's a P-type ATPase that uses the energy of ATP cleavage to deliver proton secretion from parietal cells in the gastric cavity against an electrochemical gradient. PPIs are a class of substituted benzopyridines that are transformed into active sulfonamides in the stomach's acid medium by interacting with cysteine residues in the pump. As a result, PPIs are acid-activated prodrugs that are transformed into drugs within the body.
Q13) Explain the Phenomenon of Enzyme inhibition.
A13)
- Injection
Because the chemical is injected directly into the body, injection is the sole form of exposure for poisons in which the complete amount exposed is absorbed independent of the chemical administered. Chemicals can be injected intravenously (directly into a vein), intramuscularly (straight into a muscle), subcutaneously (under the skin), or intraperitoneally (straight into the abdominal cavity) (within the membrane lining the organs of the abdomen). Intravenous injection is the fastest way to introduce a chemical into the body since blood is the vehicle for chemical distribution in the body. Because of the near-instantaneous dispersion and irreversibility, intravenous injection is a risky type of chemical exposure, with a good probability of producing drug overdose if done incorrectly.
- Ingestion
The most common way to be exposed to harmful substances is by ingestion. The degree to which a chemical is ionised is significant in deciding whether it is absorbed because most compounds diffuse over the cell membrane in their nonionized state. When the pH of the environment changes, organic acids and bases dissociate into their ionised forms. In an acidic environment (such as the stomach), organic acids are nonionized and so diffuse over a membrane, whereas organic bases are nonionized and hence diffuse across a membrane in a basic environment (such as in the intestine).
Q14) What are Nucleic Acids? Explain With Diagram.
A14) Nucleic acid is a naturally occurring chemical molecule that can be broken down to produce phosphoric acid, sugars, and an organic base combination (purines and pyrimidines). Nucleic acids are the cell's principal information-carrying molecules, and they determine every living thing's inherited features by directing the process of protein synthesis. Deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) are the two main types of nucleic acids (RNA). The genetic material in all free-living organisms and most viruses is made up of DNA, which is the master blueprint for life. RNA is the genetic material of some viruses, but it may also be present in all living cells, where it plays a key part in processes like protein synthesis.
Q15) Explain the process of Biosynthesis and degradation in nucleotides.
A15) Nucleotides are made in the cell from easily available precursors. The pentose phosphate pathway is used to make the ribose phosphate part of both purine and pyrimidine nucleotides. The six-atom pyrimidine ring is first formed, then linked to the ribose phosphate. Purines' two rings are formed as adenine or guanine nucleosides are assembled, while coupled to the ribose phosphate. The end product in both circumstances is a nucleotide with a phosphate bonded to the sugar's 5′ carbon. Finally, a kinase enzyme adds two phosphate groups to ribonucleoside triphosphate, the immediate precursor of RNA, utilising adenosine triphosphate (ATP) as the phosphate donor. To make deoxyribonucleoside diphosphate, the 2′-hydroxyl group is removed from ribonucleoside diphosphate. Another kinase then adds another phosphate group from ATP to make deoxyribonucleoside triphosphate, the immediate precursor of DNA.
RNA is regularly generated and destroyed down during regular cell metabolism. Several salvage processes reuse purine and pyrimidine residues to create additional genetic material. Purine is recovered as the matching nucleotide, whereas pyrimidine is recovered as the nucleoside.
Q16) Explain the role of DNA in cells. Describe Properties Of DNA.
A16) DNA is the genetic material that can be found in all living things, from single-celled bacteria to multicellular animals like you and me. DNA is present in the nucleus, a specialised, membrane-bound vault in the cell, as well as in some other types of organelles in eukaryotes, such as plants and animals (such as mitochondria and the chloroplasts of plants). Although DNA is located in a specialised cell area termed the nucleoid in prokaryotes, such as bacteria, it is not covered in a membrane envelope. DNA is often divided up into a number of very long, linear segments called chromosomes in eukaryotes, but chromosomes in prokaryotes, such as bacteria, are much smaller and often circular (ring-shaped). A chromosome can have tens of thousands of genes, each of which gives instructions on how to manufacture a specific product that the cell requires.
Properties of DNA
Chains of deoxyribonucleic acid, or DNA, are usually found in a double helix, which is a configuration in which two complementary (matching) chains are locked together. The sugars and phosphates on the exterior of the helix make up the backbone of DNA, which is frequently referred to as the sugar-phosphate backbone. The nitrogenous bases extend into the interior in pairs, like the steps of a staircase; each pair's bases are linked by hydrogen bonds. The helix's two strands flow in opposing directions, so one strand's 5′ end is hooked up with the 3′ end of its matching strand. (This is known as antiparallel orientation, and it is critical for DNA copying.)
Q17) Describe the properties of RNA.
A17) Ribonucleic acid (RNA) is normally single-stranded. Ribose (the five-carbon sugar), one of the four nitrogenous bases (A, U, G, or C), and a phosphate group make up an RNA nucleotide. We'll look at messenger RNA (mRNA), ribosomal RNA (rRNA), transfer RNA (tRNA), and regulatory RNAs, which are the four major forms of RNA. RNA transforms DNA into proteins to carry out biological operations, while DNA provides the code for the cell's actions.
Q18) What are Nitrogenous Bases and Sugar?
A18)
Nitrogenous bases
Organic (carbon-based) compounds with nitrogen-containing ring structures make up the nitrogenous bases of nucleotides.
Adenine (A), guanine (G), cytosine (C), and thymine (T) are the four nitrogenous bases that can be found in DNA (T). Purines, such as adenine and guanine, have two joined carbon-nitrogen rings in their architecture. In contrast, cytosine and thymine are pyrimidines with a single carbon-nitrogen ring. Adenine, guanine, and cytosine bases can also be found in RNA nucleotides, but instead of thymine, they have uracil, a pyrimidine base (U). Each base has its own structure, as illustrated in the diagram, with its own collection of functional groups connected to the ring structure.
Sugars
DNA and RNA nucleotides have somewhat distinct sets of bases, as well as slightly different sugars. Deoxyribose is the five-carbon sugar in DNA, while ribose is the sugar in RNA. These two have relatively similar structures, with one exception: ribose's second carbon has a hydroxyl group, whereas deoxyribose's comparable carbon has a hydrogen.
Q19) Explain Structure and of Nucleosides and properties of Pentose Sugar.
A19) Structure of Nucleosides
• A nucleoside has only a nitrogenous base and a five-carbon sugar, whereas a nucleotide has a nucleobase, a five-carbon sugar, and one or more phosphate groups.
• Nucleosides include cytidine, uridine, adenosine, guanosine, thymidine, and inosine, which are all connected to ribose or deoxyribose via a beta-glycosidic bond at the 1' position.
Properties of Pentose Sugars
• Ribose is the most prevalent pentose, with one oxygen atom connected to each carbon atom, and is a monosaccharide with five carbon atoms.
• Deoxyribose sugar is made by removing one oxygen atom from ribose sugar.
• Intramolecular hemiacetals are formed when the aldehyde functional group in carbohydrates reacts with neighbouring hydroxyl functional groups.
• The resulting ring structure is called a furanose because it is connected to furan.
• The ring opens and closes spontaneously, permitting rotation around the bond between the carbonyl group and the surrounding carbon atom, resulting in two different configurations ( and ). Mutarotation is the name given to this process.
Q20) Differentiate between Nucleotide and Nucleoside.
A20)
Nucleotide | Nucleoside |
A phosphate group, a sugar, and a nitrogenous base make up a nucleotide's chemical makeup. | A A sugar and a base lacking the phosphate group make up a nucleoside's chemical composition. |
They are still one of the leading causes of cancer-causing chemicals today. | They are largely employed in medicine to combat infections and cancer-causing substances. |
Adenosine, guanosine, and other nucleotides are examples of nucleotides. | Some of the most common nucleosides are nucleotides with phosphate groups added. |