Unit - 3
Lipids
Q1) What are lipids? What do they consist?
A1) Lipids are hydrocarbon-containing compounds that are essential to the structure and function of living cells. Lipids include fats, oils, waxes, certain vitamins (such as A, D, E, and K), hormones, and the non-protein portion of the cell membrane.Lipids are non-polar and so insoluble in water, but they are soluble in non-polar solvents such as chloroform.
Lipids are mostly made up of reduced hydrocarbons, which make them an efficient type of energy storage because when digested, the hydrocarbons oxidise and release enormous amounts of energy. A triglyceride, an ester made up of glycerol and three fatty acids, is the type of lipid found in fat cells for this purpose.
Q2) Where Do lipids Come from (source of it)?
A2) Excess carbohydrates in the diet are converted to triglycerides in the endoplasmic reticulum, which involves the creation of fatty acids from acetyl-CoA in a process known as lipogenesis. In animals and fungi, most of these functions are handled by a single multifunctional protein, whereas bacteria use numerous enzymes. Some unsaturated fatty acids, such as omega-3, cannot be produced in mammalian cells and must thus be obtained through the diet.
Acetyl-CoA also plays a role in the mevalonate pathway, which produces a variety of isoprenoids, including essential lipids like cholesterol and steroid hormones.
Q3) What is Hydrogenation? What are trans-fat?
A3) Hydrogenation is the addition of hydrogen gas to an oil or fat to change its melting point. The hydrogen injected forms a connection with the available carbon, converting liquid oil to solid fat. This is useful because it allows lipids to be used in a variety of ways.
A chemical process known as "partial hydrogenation" produces trans-fat. This is the process of turning liquid oil into solid fat. Trans fat, like saturated fat, has been shown to elevate LDL or "bad" cholesterol levels, thereby increasing your risk of heart disease. Trans fat, like saturated fat, reduces HDL or "good" cholesterol. A low level of HDL cholesterol is also linked to an increased risk of heart disease.
Q4) Explain the Isomerization Reaction.
A4) Isomerization (molecular structure rearrangement) of unsaturated fatty acid radicals to generate isooleic, isolinoleic, and related groups. These isomers contribute to the hardening effect by having higher melting points than natural acids. Natural oils have a cis unsaturation, which means hydrogen atoms are on one side of a plane that cuts through the double bond and alkyl groups are on the other. Some unsaturation is changed to the trans configuration during hydrogenation, with similar groups on opposite sides of the plane. The melting point of the trans isomers is substantially greater than that of the normal cis form. There is a migration of double bonds throughout the chain as some of the unsaturation is changed to the trans conformation. As a result, isomers of oleic acid can be made with the double bond anywhere between carbon atoms 2 and 17. The melting point of many of these isomerized acids is higher than that of natural oleic acid. Infrared analysis can be used to quantify the changes that occur during hydrogenation.
Q5) What is Saponification? What is Saponification Value and how to calculate?
A5) Saponification is the process of creating soap. The soap-making process, also known as saponification.
The process of creating soap is known as saponification. Soaps are long-chain fatty acid potassium or sodium salts. The ester reacts with an inorganic base to produce alcohol and soap during saponification. It happens when triglycerides are treated with potassium or sodium hydroxide (lye) to form glycerol and fatty acid salt, often known as "soap."
The saponification value is the number of mg of potassium hydroxide required to neutralise the fatty acids formed when 1 g of the substance is completely hydrolyzed.

Q6) Describe the procedure for determination of Acid Value.
A6) The acid value is the amount of potassium hydroxide needed to neutralise one gramme of the substance's free acid.
In a 250-mL flask, accurately weigh roughly 10 g of the substance, or the amount stated in the monograph, and add 50 mL of a combination of equal volumes of ethanol (750 g/l) TS and ether R, neutralised with potassium hydroxide (0.1 mol/l) VS following the addition of 1 mL of phenolphthalein/ethanol TS. If necessary, heat until the item is completely dissolved, then cool; titrate with potassium hydroxide (0.1 mol/l) VS, constantly shaking the contents of the flask until a pink colour is obtained that lasts 15 seconds. Take note of the needed mL. (a). Calculate the acid value using the formula below:
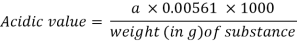
Q7) What is Iodine Number? Explain the procedure for calculating iodine value?
A7) Iodine number is a measure of the degree of unsaturation of an oil, fat, or wax; it is the quantity of iodine, in grammes, taken up by 100 grames of the oil, fat, or wax. Iodine is not taken up by saturated oils, fats, or waxes, therefore its iodine value is 0; nevertheless, iodine is taken up by unsaturated oils, fats, and waxes. (Unsaturated compounds have molecules with double or triple bonds, which are iodine reactive.)
In a dry 300-mL to 500-mL stoppered flask, place an amount of the test substance, properly weighed, as described in the monograph, add 15 mL of carbon tetrachloride R, and dissolve. Unless otherwise specified in the monograph, add 25 mL of iodine bromide TS, insert the stopper, previously moistened with potassium iodide (80 g/l) TS, gently shake the flask, and keep in the dark for 30 minutes. Add 20 mL potassium iodide (80 g/l) TS and 150 mL water to a flask and titrate with sodium thiosulfate (0.1 mol/l) VS while shaking the contents of the flask, using starch TS as an indicator at the conclusion of the titration. Take note of the needed mL. (a). Simultaneously, perform the operation in the same way but without the chemical being tested, and record the number of mL of sodium thiosulfate (0.1 mol/l) VS necessary (b). Calculate the iodine value using the formula below.
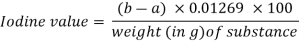
Q8) Explain the Mechanism of Oil Reversion.
A8) The fundamental mechanism involved in oil reversion is oxidation. Linolenic acid is ten times more sensitive to oxidation than linoleic acid and one hundred times more sensitive than oleic acid. Double bond oxidation is a radical-driven reaction.
Radical reactions typically have three steps:
1. An energy source (heat; light) creates a radical on the fatty acid in the initiating process.
2. An oxygen propagation phase produces peroxides, which react with additional unsaturated fatty acids to produce new radicals.
3. An interaction between two radicals that results in the formation of a new single bond.
Q9) Explain the Types Of Rancidity.
A9) There are two types of rancidity:
1. Oxidative Rancidity:
“Oxidative rancidity” refers to rancidity that occurs as a result of oxygen degradation to foods. During the process, oxygen molecules interact with the oil's natural structure, altering its odour, taste, and safety for consumption, i.e. fat is oxidised and decomposes into compounds with shorter carbon chains, such as fatty acids, aldehydes, and ketones, all of which are volatile and contribute to the rancid fat's unpleasant odour. The development of both unpleasant and dangerous chemicals is caused by oxidative rancidity.
2. Hydrolytic Rancidity:
Enzymatic hydrolysis of fats results in the release of free fatty acids from glycerides, resulting in a rotten odour. This is known as hydroiytic rancidity. In glycerides, hydrolysis separates fatty acid chains from the glycerol backbone. Further auto-oxidation of these free fatty acids results in oxidative rancidity.
Q10) Explain the Factors which affect Rancidity and Reversion.
A10)
1. Oxidation:
Because lipids are eight times more soluble in oxygen than water, the oxidation that results from this exposure is the primary source of rancidity. Unsaturated fats are oxidised largely through a free radical-mediated mechanism. In rancid foods and oils, these chemical processes can produce highly reactive molecules, which are responsible for the unpleasant and toxic aromas and flavours. Auto-oxidation, often known as oxidative rancidity, is the name given to this process.
2. Hydrolysis:
Under the right conditions, triglycerides react with water to generate diglycerides and free fatty acid residues. Monoglycerides and fatty acids are formed when diglycerides mix with water. Finally, the monoglycerides hydrolyzed entirely, yielding glycerol and fatty acids. This is known as hydrolytic rancidity.
3. Presence of Microorganisms – Microbial Lipase:
Lipase is a hydrolytic enzyme produced by certain bacteria that directly interferes with the breakdown of triglycerides to create glycerols and fatty acids. These fatty acids become rancid due to auto-oxidation.
4. Presence of Unsaturation in Fatty Acid Chain:
When unsaturated components of a fatty material are exposed to air, they are transformed to hydroperoxides, which then break down into volatile aldehydes, esters, alcohols, ketones, and hydrocarbons, some of which have unpleasant odours. The aforementioned process, as well as hydrolysis, which releases volatile and malodorous acids, mainly butyric acid, causes butter to get rancid. Saturated fats, such as beef tallow, resist oxidation and turn rancid at room temperature.
5. Polyunsaturation:
The higher a fat's polyunsaturation, the faster it will go rancid. Animal fats must become several times more rancid than vegetable oils. Oils and fats with polyunsaturated fatty acids are more prone to rancidity than monosaturated and other forms of saturated fatty acids.
6. Chemical Structure of Oils and Fats:
Oils and fats that are chemically more complex and include a greater number of double bonds, carboxyl or hydroxyl groups have a greater likelihood of becoming rancid. Auto-oxidation is aided by the double bonds found in fats and oils. Auto-oxidation is particularly common in oils with a high degree of unsaturation.
7. Temperature and pH:
These are the major factors that cause fat- and oil-rich foods to get rancid. The hydrolytic action of microbial lipase requires a specific temperature and alkaline pH. The auto-oxidation and hydrolysis are influenced by temperature and pH in an indirect manner.
8. Heat and Light:
Heat and light speed up the rate at which lipids react with oxygen, i.e. heat speeds up auto-oxidation. The formation of free radicals in rancidity and reversion of oils and fats is fueled by heat and light.
Q11) Explain the methods for Prevention of Rancidity.
A11) Rancidity can be prevented by several ways which are mentioned briefly:
1. Addition of Antioxidants:
Antioxidants are the most effective way to keep food from becoming rancid. Antioxidants are added to fat-containing foods in order to retard the development of rancidity due to oxidation.
There are five types of antioxidants:
(1) Natural antioxidants.
(2) Synthetic antioxidants.
(3) Semi-synthetic antioxidants – gallic acid, propylgallate.
(4) Metal chelators – citric acid, phosphoric acid.
(5) Oxygen scavengers – ascorbic acid.
2. Addition of Sequestering Agents:
Metals are bound by sequestering agents, which prevent them from initiating auto-oxidation. EDTA (ethylene diamine tetra acetic acid) and citric acid are examples of sequestering agents.
3. Proper Storage of Fats and Oil Food:
Another strategy to prevent food from becoming rancid is to store it properly, away from the effects of oxygen. Because heat and light accelerate the rate of reactivity of lipids with oxygen, rancidification can be reduced by storing fats and oils in a cold, dark environment with little exposure to oxygen or free radicals.
Q12) Differentiate Between Catabolism and Anabolism.
A12)
Catabolism | Anabolism |
Catabolism is the breakdown of large, complex compounds into smaller, more absorbable molecules. | Anabolism is the process of constructing molecules that are essential for the body's proper functioning. |
Catabolism is a metabolic process that releases energy. | Energy is required for anabolic processes. |
Adrenaline, cytokine, glucagon, and cortisol are hormones that are involved in the processes. | Estrogen, testosterone, growth hormones, and insulin are all involved in the process. |
Proteins decompose into amino acids, glycogen decomposes into glucose, and triglycerides decompose into fatty acids are all examples of catabolic processes. | Polypeptides are made from amino acids, glycogen is made from glucose, and triglycerides are made from fatty acids. |
Potential energy is converted to kinetic energy during catabolism. | Kinetic energy is turned into potential energy during anabolism. |
It is necessary for living entities to carry out a variety of tasks. | It's necessary for upkeep, growth, and storage. |
Q13) Use the information in this nutrition facts label to determine the amount of Calories (Cal = kcal) and kilojoules (kJ) from fat, carbohydrates and protein in the snack mix.
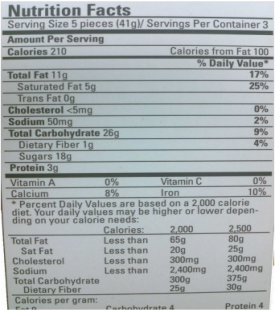
A13) Known
Energy per gram
- Fat = 9 kcal/g (Cal/g)
- Carbohydrate = 4 kcal/g (Cal/g)
- Protein = 4 kcal/g (Cal/g)
Grams
- Fat = 11 g
- Carbohydrate = 12 g
- Protein = 5 g
The label specifies the number of grammes of each energy source (fat, carbohydrate, and protein) as well as the amount of energy per gramme of each energy source. To get the Energy, multiply the grammes by the Energy per gramme. The conversion factor is the energy per gramme. Because you want your final answer to be energy in Cal, kcal, or kJ, it should be multiplied such that the energy is on top and the grammes are on the bottom.
Step 2: Solve
Energy from Fat:
11 g x 9 kcal/g = 99 kcal = 99 Cal
Convert to kJ
99 Cal x 4.184kJ/Cal = 414.216 kJ= 414kJ
Energy from Carbohydrate:
12 g x 4 kcal/g = 48 kcal = 48 Cal
Convert to kJ
48 Cal x 4.184kJ/Cal = 200.832 kJ = 201 kJ
Energy from Protein:
5 g x 4 kcal/g = 20 kcal = 20 Cal
Convert to kJ
20 Cal x 4.184kJ/Cal = 83.68 kJ = 84 kJ
Q14) Enlist the Different test for Carbohydrates and protein.
A14) Test for Carbohydrates:
- Molisch’s test – Given sample food + Molisch’s reagent → Purple or violet ring confirms the presence of carbohydrate.
- Fehling’s test – Given sample food + Fehling’s reagent → Red precipitate confirms the presence of carbohydrates
- Benedict’s test – Given sample food + Benedict’s reagent → Red precipitate confirms the presence of carbohydrates.
- Tollen’s test – Given sample food + Tollen’s reagent → Silver mirror confirms the presence of carbohydrates.
Iodine test – Given sample food + Iodine solution → Blue colour solution confirms the presence of starch
Test for Proteins:
- Biuret test – Given sample food + Aqueous copper sulfate → Violet colouration confirms the presence of Proteins
- Xanthoproteic test – Given sample food + Nitric acid → Yellow colour solution confirms the presence of proteins.
- Millions test – Given sample food + Mercuric sulfate in the presence of sodium nitrite and sulfuric acid → Brick red colour solution confirms the presence of proteins.
- Ninhydrin test – Given sample food + Pyridine solution of ninhydrin → Violet colour solution confirms the presence of proteins.
Q15) What are the different test for oils and fats?
A15) Test for Oils and Fats:
- Solubility test – Given sample food + Chloroform or alcohol → Miscible with chloroform and immiscible with water the fat presence is confirmed.
- Translucent spot test – Given sample food + rubbed between the folds of filter paper → presence of translucent spot then the presence of fats is confirmed.
Acrolein test – Given sample food + Potassium bisulfite KHSO4 → Pungent irritating odor then the presence of fats or oil is confirmed
Q16) Explain the different types of Fats.
A16) There are several types of fat:
- Monounsaturated
- Polyunsaturated
- Saturated
Saturated fats have a higher proclivity for raising cholesterol levels and increasing the risk of atherosclerosis. Saturated fats, which are solid at room temperature, are typically found in animal-derived foods. Monounsaturated and polyunsaturated fatty acids, which are liquid at room temperature, are often found in plant fats. The exceptions are palm and coconut oil. They have a higher percentage of saturated fats than other plant oils.
Trans fats (also known as trans fatty acids) are a type of fat. They are created by adding hydrogen atoms to monounsaturated or polyunsaturated fatty acids (hydrogenation). Hydrogenated fats can be partially or completely hydrogenated (or saturated with hydrogen atoms). Trans fats have been shown to raise cholesterol levels in the body and increase the risk of atherosclerosis.
Q17) Explain how Cells obtain energy by the oxidation of organic molecules.
A17) All animal and plant cells are powered by energy stored in the chemical bonds of organic molecules, whether these be sugars that a plant has photosynthesized as food for itself or the mixture of large and small molecules that an animal has eaten. Organisms must extract this energy in an useful form in order to utilise it to live, grow, and reproduce. Energy is taken from food molecules by a process of slow oxidation, or controlled burning, in both plants and animals.
The atmosphere of the Earth contains a lot of oxygen, and in the presence of oxygen, the most energetically stable form of carbon is CO2, while the most energetically stable form of hydrogen is H2O. A cell can obtain energy from sugars or other organic molecules by combining their carbon and hydrogen atoms with oxygen to form CO2 and H2O, respectively, a process known as respiration.
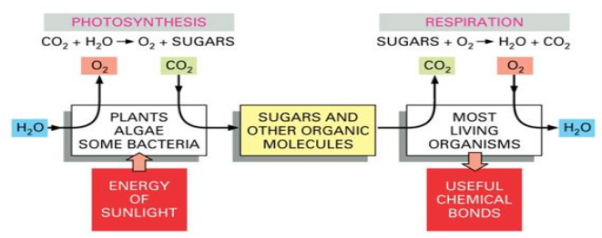
Q18) What is Catabolism? What is cellular respiration?
A18) Catabolism refers to a group of biological reactions that break down large compounds into smaller ones. Cells use catabolic processes to create energy or fuel anabolism because they are thermodynamically advantageous and spontaneous. Catabolism is an exergonic process that produces heat through hydrolysis and oxidation. Cells can store important raw materials in complex molecules, break them down by catabolism, and reuse the smaller molecules to create new products. Protein, lipid, nucleic acid, and polysaccharide catabolism, for example, produces amino acids, fatty acids, nucleotides, and monosaccharides, respectively. Carbon dioxide, urea, ammonia, acetic acid, and lactic acid are some of the waste products that are produced.
Cellular respiration is a catabolic process that breaks down glucose into useful energy for a cell.
The entire cellular respiration reaction is:
C6H126O2 + 6O2 6CO2 + 6H2O + energy
Cellular respiration, like all catabolic processes, releases energy that can be harnessed and utilized by other reactions in the cell
Q19) Explain the Oxidation and Reduction Reaction.
A19) Oxidation refers to the removal of electrons, while reduction refers to the addition of electrons. Oxidation and reduction always happen at the same time, which means that if one molecule receives an electron in a reaction (reduction), another molecule loses an electron (oxidation). When a sugar molecule is oxidised to CO2 and H2O, the O2 molecules involved in the formation of H2O gain electrons and are thus reduced.
When a molecule in a cell accepts an electron (e-), it frequently also accepts a proton (H+) (protons being freely available in water). In this situation, the overall result is to add a hydrogen atom to the molecule:
A + e- + H+ → AH
It's very simple to detect if an organic molecule is being oxidised or reduced: reduction occurs when the number of C-H bonds in the molecule grows, whereas oxidation occurs when the number of C-H bonds in the molecule drops
Q20) What are Enzymes and Activation Energy?
A20) A catalyst is a substance that aids the occurrence of a chemical reaction, and enzymes are the molecules that catalyse biochemical events. The majority of enzymes are proteins that play an important role in decreasing the activation energy of chemical processes within the cell. Enzymes accomplish this by attaching to the reactant molecules and keeping them in a position that facilitates the chemical bond-breaking and formation processes. It's vital to remember that whether a reaction is exergonic (spontaneous) or endergonic is unaffected by enzymes. This is due to the fact that they have no effect on the free energy of the reactants or products. They just lower the activation energy necessary to proceed with the reaction. Furthermore, the reaction that an enzyme catalysis has no effect on the enzyme itself. After catalysing one reaction, the enzyme is free to participate in subsequent processes.
