Unit - 4
Pharmaceutical Compounds: Structure and Importance
Q1) Classify Drug on Basis of Drug Action, chemical Structure, Molecular Target.
A1) Classification of Drugs on the basis of Drug Action:
• Varied medications have different ways of eliciting a reaction, which is referred to as drug action.
• Drug action is more defined in terms of how it causes a response. There are several drugs to treat hypertension, for example, but each type of drug has a different function.
• All hypertension medications lower blood pressure, although in different ways.
Classification of Drugs on the basis of Chemical Structure:
• This is a frequent drug classification. Drugs with the same drug action and pharmacological effect usually have the same fundamental skeletal structure with minor branching variations. As a result, certain medications have greater potential than others. Sulphonamides, for example, all have the same skeleton structure.
Classification of Drugs on the basis of Molecular Targets:
• Drugs target macromolecules inside the body to elicit a biological reaction, which are known as target molecules or drug targets. The target of drugs with the same mechanism of action will be the same. This method of medication classification is more useful during clinical trials.
Q2) Explain Chemical Stability of Pharmaceutical Organic Compound Penicillin?
A2) Hydrolytic enzymes like penicillin acylase, obtained from E. Coli, have been shown to be capable of catalysing the hydrolysis of natural penicillin to create 6-aminopenicillanic acid. In the semi-synthetic manufacture of antibiotics, this acid is a crucial step. The serine hydroxyl group is converted into an ester group, according to experimental findings and structural studies of the enzyme. Penicillin is known to be sensitive to acidic environments, which catalyse its decomposition. The amide bond is broken when the acid reacts with the nitrogen intra-cyclic atom. The breaking is facilitated by the extra cyclic nitrogen atom, which donates its non-bonded electrons to the secondary chain carbonyl group, which then donates its electrons to the -lactam ring, resulting in the formation of a new ring and the breaking of the -lactam ring, resulting in the complete destruction of penicillin. Penicillinase has also been discovered to be a -lactamase enzyme that degrades penicillin by initiating the hydrolysis process of the -lactam ring to produce penicillic acid. In the presence of metallic ions such as zinc (II) and cadmium (II), four conventional penicillins, such as amoxicillin, ampicillin, penicillin G, and penicillin V, can be degraded in methanol. The Lewis acid activates the lactam ring carbonyl group, enhancing the electrophilic nature of the activated lactam ring carbonyl group's carbon atom, and methanol reacts as the nucleophile.
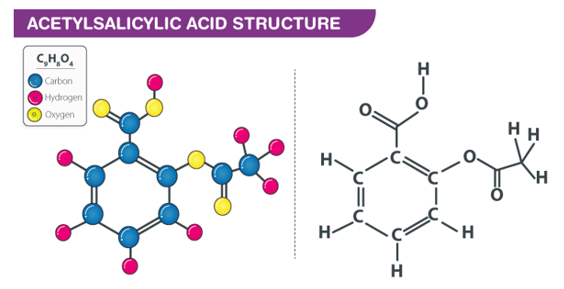
Q3) Explain the Structure of Aspirin.
A3) Aspirin provides a number of advantages, ranging from pain relief to lowering the chance of catastrophic illnesses including heart attacks and strokes. Aspirin is a widely used medication. It comes in a variety of forms, including capsules, water-soluble pills, powders, and oral gels.Aspirin (acetylsalicylic acid) is an anti-inflammatory and analgesic medication used to treat cardiovascular disease. The observation that thrombocytopenia-inducing drugs reduced metastases led to the investigation of aspirin as an anticancer treatment, with aspirin significantly reducing fibrosarcoma metastasis in animal models.
Aspirin's structure is shown below.
Q4) Explain the Therapeutic Uses of Aspirin.
A4) Aspirin (acetylsalicylic acid) is a prescription drug used to relieve pain and inflammation. It's a type of non-steroidal anti-inflammatory medication. It's also used to prevent blood clots, heart attacks, and strokes, as well as bowel cancer. While some studies have indicated that aspirin helps reduce the incidence of heart attacks and cancers of the intestines, stomach, and oesophagus, doctors are advised to use caution when taking aspirin as a preventive therapy because it might cause gastrointestinal bleeding and damage.
People with renal failure, liver disease, or haemophilia should consult a doctor before using aspirin. Some people use aspirin to get euphoric or as a form of self-harm by taking more than the recommended amount.
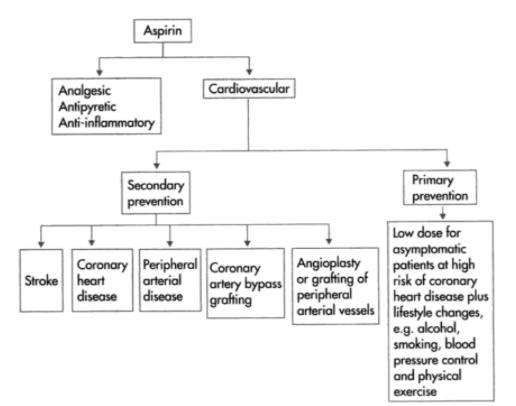
Aspirin increases the risk of bleeding in the liver, small intestine, and brain. The lining of your intestine and gut is generally protected from the acid in your stomach by a membrane. If aspirin is taken in high doses and for a long time, it will eventually harm this layer. The bleeding will be exacerbated by this damage. The use of aspirin to prevent blood clots will also hinder the normal mending of broken blood vessels and increase the risk of bleeding in the brain.
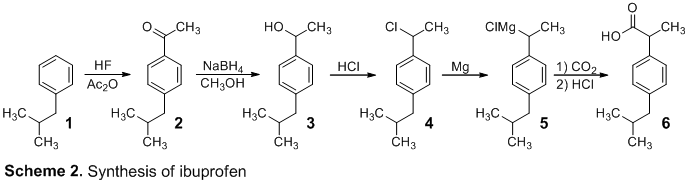
Q5) What is ibuprofen? Explain Ibuprofen Synthesis?
A5) Ibuprofen is an NSAID, or nonsteroidal anti-inflammatory drug, that has analgesic, fever reducing, and anti-inflammatory actions in larger dosages. Ibuprofen is listed as an essential drug by the World Health Organization (WHO)Trusted Source. The list identifies the bare minimum of medical requirements for a basic healthcare system.
Steroids and narcotics, or opioids, are two other sorts of pain relievers. Long-term steroid use can have serious side effects, and using opioids can lead to misuse. NSAIDs are safer than both of these NSAIDs such as ibuprofen, aspirin, and naproxen are well-known, in part because they are available over-the-counter at pharmacies.
By inhibiting the production of cyclooxygenase (COX)-1 and COX-2, ibuprofen lowers pain, fever, edoema, and inflammation. These compounds are released by the body in reaction to illness and injury.
When taking ibuprofen by mouth, the effects should be noticeable after 20–30 minutes.
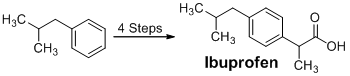
Ibuprofen Synthesis:
Ibuprofen was created using isobutylbenzene as a starting material. Friedel-Crafts acylation, reduction, chloride substitution, and the Grignard reaction were all used in the synthetic process. IR and 1H NMR spectroscopy were used to examine the products of each step, and melting point analysis was used to confirm the final product.
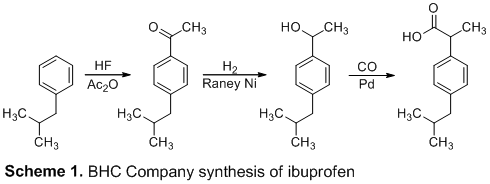
A five-step ibuprofen synthesis that resembles the industrial BHC synthesis Our synthesis started with a Friedel-Crafts acylation and carbonyl reduction, same as the BHC technique. However, because the commercial technique, which uses carbon monoxide (CO) at 500 psi, was avoided due to safety concerns, we used a chloride substitution, Grignard formation, and Grignard reaction in the last steps.
Q6) What is Chloramphenicol and how does it work?
A6) Chloramphenicol is sold under several brand names, including Chloramphenicol IV and Chloromyectin. In the United States, these brand names have been phased out. Chloramphenicol Doses:
Injectable remedy
Considerations for Dosage - Give as follows:
Susceptible Strains Cause Serious Infections
Those who are adults:
50 mg/kg/day intravenously divided every 6 hours; in rare circumstances, patients with moderately resistant organisms or severe infections may need a higher dose of up to 100 mg/kg/day; reduce these high dosages as soon as possible.
Infections that affect the entire body
Children's health:
- Infants and children: When appropriate cerebrospinal fluid concentrations are sought, up to 100 mg/kg/day may be required; however, dose should be reduced to 50 mg/kg/day as soon as possible.
- Infants and children with suspected immature metabolic functions: 25 mg/kg/day divided every 6 hours should result in therapeutic medication concentrations in the blood.
- Neonates are children who are born prematurely (Infants younger than 28 days
- Children's health
- Loading dosage (LdD): 20 mg/kg intravenously once; maintenance dosage should be given 12 hours following the loading dose.
- Dose for Maintenance
- Infants under the age of 7 days: 25 mg/kg intravenously every 24 hours.
- Infants weighing less than 2000 g and older than 7 days: 25 mg/kg/day intravenously every 24 hours
- Infants over 7 days old and weighing more than 2000 g: 50 mg/kg/day administered intravenously every 12 hours
- Troughs 5-10 mg/l, peaks 10-20 mg/l
- Other Indications and Applications
- Those who are adults:
- Only use as a last resort for meningitis, typhoid, or rickettsial infection.
Q7) Explain How Curcumin Is an Anti-Inflammatory? And how prevent Cancer?
A7) Turmeric's greatest claim to fame is that it's often used to combat inflammation, and curcumin is responsible for the majority of turmeric's anti-inflammatory properties. According to a previous study, curcumin may be a more effective anti-inflammatory treatment than conventional anti-inflammatory drugs like Advil (ibuprofen) and aspirin in the proper amount. Curcumin may aid in the treatment of inflammatory bowel disease, pancreatitis, and arthritis, as chronic inflammation plays a role in many chronic diseases.
Cancer treatment:
As inflammation is linked to tumor growth, anti-inflammatory compounds such as curcumin may play a role in treating and preventing a variety of cancer types, including colorectal, pancreatic, prostate, breast, and gastric cancers In fact, research in mice suggests that curcumin may help slow the spread of tumor cells and may even prevent tumors from forming in the first place. It may do this in several ways, including disrupting the formation of cancerous cells at various stages in the cell cycle, interfering with cell signaling pathways, and even causing those cancerous cells to die
Q8) How Curcumin Protect Against Heart Disease?
A8) Curcumin may improve endothelial function, or the health of the thin membrane that covers the inside of the heart and blood vessels. This membrane plays a key role in regulating blood pressure. Lower endothelial function is associated with aging and an increased risk of heart disease. Thus, curcumin may help protect against age-related loss of function and reduce your likelihood of developing heart disease.
In one study, researchers compared the effects of an eight-week aerobic exercise program and a curcumin supplement in improving endothelial function in postmenopausal women. Both the exercise and the curcumin group saw equal improvements in endothelial function, whereas the control group saw no changes.
Another study found that curcumin was equally effective at improving endothelial function in people with type 2 diabetes (heart disease is a common comorbidity of type 2) as the drug Lipitor (atorvastatin), a medication commonly prescribed to reduce the risk of heart attack and stroke
Q9) Explain the compound Azadirachtin (Neem).
A9) Azadirachtin is a group of compounds. It is a mixture of related substances extracted from neem seed kernels with a complex structure. Azadirachtin is only found in the seeds. Azadirachtin has a variety of effects on insects, including serving as a growth regulator, anti-feedant, repellant, sterilant, and oviposition inhibitor. Azadirachtin is more effective on insects in their immature/young life stages than on eggs or adults. However, azadirachtin takes longer to work than other pesticides, owing to the fact that it alters or modifies insect behaviour. Azadirachtin is a stomach poison that requires insects to absorb the active ingredient while feeding in order to be poisoned. Chewing insects seemed to respond better to activity than sucking insects. This could explain why azadirachtin works so well against caterpillars. The hydrophobic extract of neem oil has been clarified. Insect and mite pests are suffocated (breathing passages are blocked) by a clarified hydrophobic extract of neem oil (neem oil). Aphids, whiteflies, spider mites, mealybugs, and scales are among the soft-bodied insect and mite pests that neem oil is particularly effective against. Eggs, immatures (larvae or nymphs), and adults may be killed by neem oil. Aphids, leafhoppers, mealybugs, mites, scales, and whiteflies are all targets for this product. Due to its vulnerability to ultra-violet light (sunlight) degradation, azadirachtin and purified hydrophobic extract of neem oil have short residual action, which necessitates repeated applications. Both compounds have a low toxicity to humans and mammals, with LD50 values of more than 5,000 mg/kg. Furthermore, azadirachtin and the clarified hydrophobic extract of neem oil are less toxic to most natural enemies (parasitoids and predators) than traditional insecticides.
Q10) Explain the Anticancerous Activity of Neem.
A10) Cancer is a complex disease that affects people all around the world. Changes in molecular/genetic pathways play a role in cancer's growth and progression. The allopathic therapy module is effective on one hand, but it has a negative impact on normal cells. Plants and their contents have previously been shown to prevent the growth of malignant cells through modulating cellular proliferation, apoptosis, tumour suppressor genes, and a variety of other molecular pathways. Flavanoids and other compounds in neem help to prevent cancer by inhibiting the growth of cancer cells. A large number of epidemiological studies suggest that a high flavonoid consumption is linked to a lower cancer risk. Neem oil contains different neem limonoids, which reduce 7,12-dimethylbenz(a)anthracene's mutagenesis effects. The cytotoxic effects of nimbolide found in leaves and flowers on human choriocarcinoma (BeWo) cells

Q11) Enlist the Drug which interact with ranitidine. Why it is used? Enlist the side effect.
A11) Ranitidine is in the drug class H2 antagonists.
- Ranitidine is used to treat the following conditions:
- Cutaneous Mastocytosis
- Duodenal Ulcer
- Duodenal Ulcer Prophylaxis
- Eczema
- Erosive Esophagitis
- Gastric Ulcer Maintenance Treatment
- Gastrointestinal Hemorrhage
- GERD
- Hiatal Hernia
- Indigestion
- Laryngopharyngeal Reflux
- Pathological Hypersecretory Conditions
- Stomach Ulcer
- Ranitidine may be used as part of a combination therapy. This means you may need to take it with other medications.
- Ranitidine is typically used for short-term treatment, especially for GERD. If you’re taking this drug for other conditions, you may need long-term treatment.
Ranitidine side effects
Drowsiness and other adverse effects are possible with ranitidine oral tablet.
More common side effects
The more common side effects of ranitidine oral tablet can include:
- Headache
- Constipation
- Diarrhea
- Nausea and vomiting
- Stomach discomfort or pain
Q12) What Are Dyes? And write structure of various dyes?
A12) The dye industry has long been associated with the advancement of synthetic organic chemistry. Henry Perkin oxidised benzenamine (aniline) with potassium dichromate and separated a purple compound that was suitable for dyeing silk from the product (which was primarily aniline black; Section 23-11D). Perkin had a difficult time getting the dye into commercial production. He had to design and build his own equipment as well as invent efficient syntheses for starting materials because there was no organic chemical industry at the time. His path to benzenamine began with coal-derived crude benzene, which he nitrated before reducing with iron and acid. Because concentrated nitric acid was unavailable, he had to produce his own (from nitrate salts and sulfuric acid). Otto Fischer did not discover the structure of Perkin's dye, known as mauveine, until 1890. Because the benzene utilised contained methylbenzene, the dye was actually a mixture), yet the result of benzenamine oxidation is structurally comparable to aniline black:
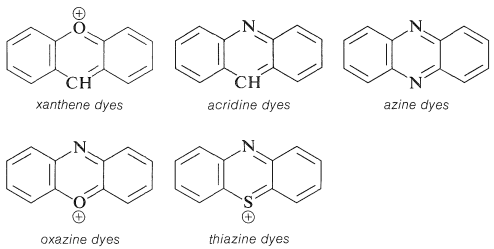
There is more to a successful dye than just an attractive color.5 If it is to be useful, say for coloring fabrics, some simple means must be available for introducing the color into the fiber and then, usually of greater difficulty and importance, the color must be reasonably permanent - that is, resistant to normal laundry or cleaning procedures (wash-fast) and stable to light (light-fast).
Q13) Explain the Mordant and Vat dyes.
A13) Mordant dye:
Mordant dye is a colourant that can be bonded to a material for which it has little or no affinity without the use of a mordant, which is a chemical that mixes with the dye and the fibre. Because dichromates and chromium complexes are the most common modern mordants, the term "mordant dye" usually refers to chrome dye. With different mordants, most mordant dyes produce distinct colours. Wool, wool blends, silk, cotton, and certain modified-cellulose fibres can all be dyed with mordant dyes.
Vat dye:
Vat dye is a broad category of water-insoluble dyes, including indigo and anthraquinone derivatives, that are employed mostly on cellulosic fibres. To impregnate the fibre, the dye is administered in a soluble, reduced form, which is then oxidised in the fibre back to its original insoluble form. Vat dyes are particularly light and washing resistant. Because vat dyes are insoluble in water, they can't be used for dyeing directly. They become soluble in an alkali and acquire affinity for cellulose fibres when reduced to a leuco form (colourless). Dyeing or printing can be done with a leuco form solution. The original insoluble dye is generated within the fibre structure during oxidation. This group of dyes includes indigo and indigosol O.
Q14) Explain the Classification of Dyes.
A14)
1. Natural Dye
Dying has been a flourishing trade since long, in different parts of the world. The dyes used in times before progress in chemical science were only natural. Dyes were derived from plants and animals. Indigo trade and farming in northern India is an example of the scale of trade.
Synthetic dyes have taken over the industry because of less cost and more reliability but natural dyes such as haematoxylin, carmine and orcein are still in use in the industry.
2. Synthetic Dye
Synthetic dyes are those that are made from organic and inorganic chemical components. Synthetic dyes include acidic, basic, azoic, nitro, vat dyes, mordant dyes, and sulphur dyes, among others.
3. Direct Dye
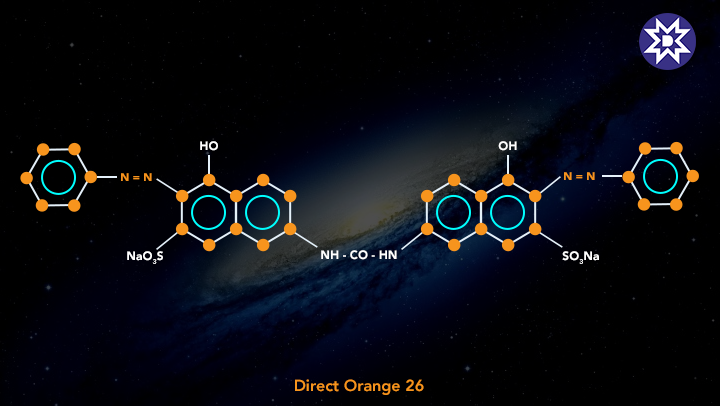
Preparing an aqueous solution and immersing the fabric in it is how these dyes are applied to the fabric. Direct dyes are used to colour fabrics that can form hydrogen bonds with the dye molecule. With the introduction of this method for cotton dying, the use of mordants or other binders became superfluous. Direct dyes are more fastidious than other dyes, but they lack the colour brilliance of other dyes. The diazotization treatment compensates for this. Direct dyes are used to dye fabrics such as cotton, linen, rayon, wool, silk, and nylon.
4. Disperse Dye
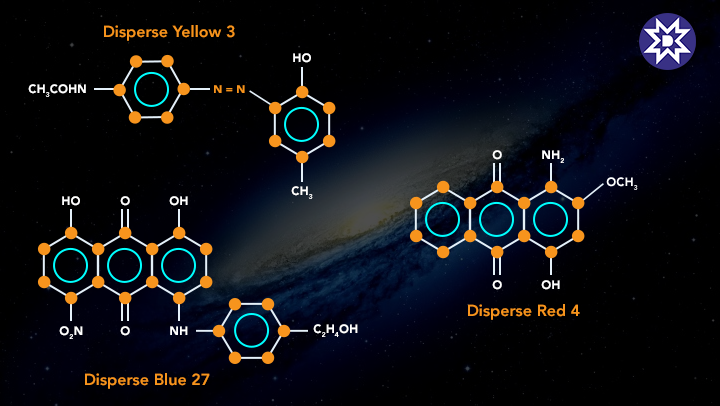
These dyes were created to colour secondary cellular acetate fibres and are relatively water insoluble. Disperse dyes are made by grinding dye into small particles and dissolving it in a solution with the help of dispersing agents. When fibre is submerged in the solution, it absorbs the colour. Disperse dyes are used to colour polyester, nylon, acetate, and triacetate fibres.
5. Reactive Dye
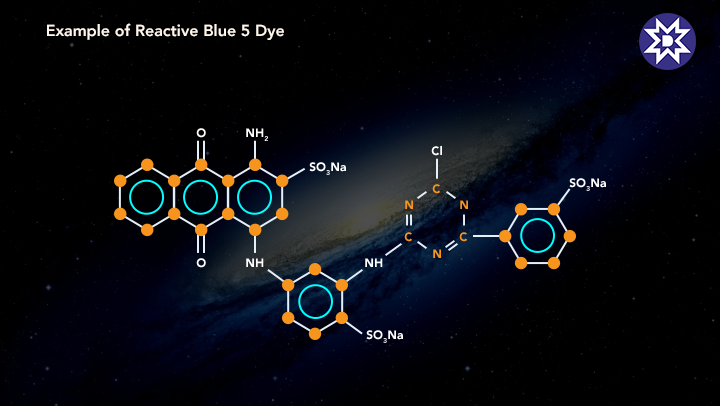
Covalent bonds are created with cellulose fibres in reactive dyes. Such connections have a high degree of fastness. It happens because strong molecular connections are difficult to break. As a result, they have a higher comparative resistance to light and washing. Read on to learn more about the benefits of reactive dyes.
6. Solvent Dye
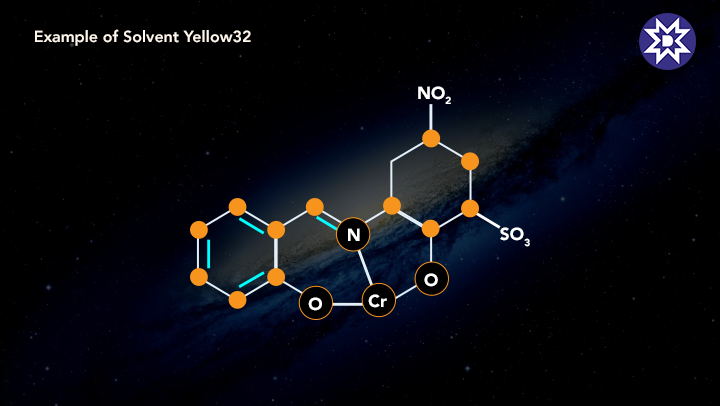
Alcohol, chlorinated hydrocarbons, and liquid ammonia are all soluble in these colours, but not water. These dyes are widely used in the petroleum sector. The dye's colours are created by dissolving it in a target, which is usually lipids or non-polar solvents. Plastics, synthetics, gasoline, oil, and waxes are all coloured with them.Due to the rising industries in emerging countries, all types of dyes are in high demand.
Q15) Explain the mechanism of diazo coupling.
A15) The diazo-coupling reaction dates back to the 1850s (and a close association with Imperial College via the first professor of chemistry there, August von Hofmann) and its mechanism was much studied in the heyday of physical organic chemistry. Nick Greeves, purveyor of the excellent ChemTube3D site, contacted me about the transition state (I have commented previously on this aspect of aromatic electrophilic substitution). ChemTube3D recruits undergraduates to add new entries; Blue Jenkins is one such adding a section on dyes.
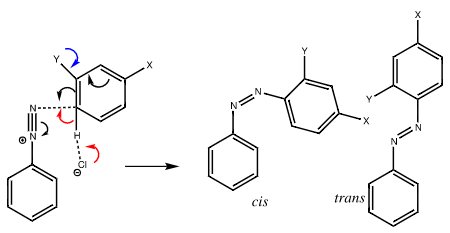
Either in the initial electrophilic attack (black arrows) or in the following proton removal, the mechanism can be rate limiting (red arrows using an intermolecular base such as chloride anion). The trans-diazo molecule is usually thought to be the product rather than the cis-diazo compound.
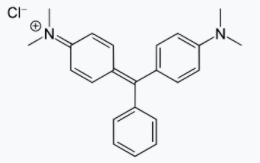
Q16) Explain the Structures and properties of Malachite green.
A16) Malachite green is an organic chemical used as a colour as well as a contentious antibiotic in aquaculture. Malachite green has been used as a dye for silk, leather, and paper for centuries. Despite its name, the dye is not made from the mineral malachite; the name is derived from the color's likeness.
Structure
Malachite green is classified in the dyestuff industry as a triarylmethane dye and also using in pigment industry. Formally, malachite green refers to the chloride salt [C6H5C(C6H4N(CH3)2)2]Cl, although the term malachite green is used loosely and often just refers to the colored cation. The oxalate salt is also marketed. The anions have no effect on the color. The intense green color of the cation results from a strong absorption band at 621 nm (extinction coefficient of 105 M−1 cm−1). Malachite green is prepared by the condensation of benzaldehyde and dimethylaniline to give leuco malachite green (LMG): C6H5CHO + 2 C6H5N(CH3)2 → C6H5CH(C6H4N(CH3)2)2 + H2O Second, this colorless leuco compound, a relative of triphenylmethane, is oxidized to the cation that is MG: C6H5CH(C6H4N(CH3)2)2 + HCl + 1⁄2 O2 → [C6H5C(C6H4N(CH3)2)2]Cl + H2O A typical oxidizing agent is manganese dioxide.
Q17) Explain Phthalein dyes- Phenolphthalein.
A17) Phenolphthalein, (C20H14O4), an organic compound of the phthalein family that is widely employed as an acid-base indicator. As an indicator of a solution’s pH, phenolphthalein is colourless below pH 8.5 and attains a pink to deep red hue above pH 9.0. Phenolphthalein, which is ordinarily colourless, turns a bright red colour when it reaches pH 10.0. Phenolphthalein is a strong laxative that takes effect in 6–8 hours and might linger for 3–4 days. It's possible that you'll get side effects including kidney irritation or a rash on your skin. Phenolphthalein was commonly used in over-the-counter laxatives until it was prohibited in 1999 by the US Food and Drug Administration because animal research suggested it could cause cancer in humans.
Q18) Explain Phthalein dyes- Fluorescein.
A18)
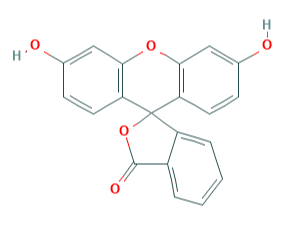
Fluorescein is a dye used in angiography or angioscopy of the iris and retina.
Fluorescein, also called Resorcinolphthalein, organic compound of molecular formula C20H12O5 that has wide use as a synthetic colouring agent. It is prepared by heating phthalic anhydride and resorcinol over a zinc catalyst, and it crystallizes as a deep red powder with a melting point in the range of 314° to 316° C (597° to 601° F). Fluorescein was named for the intense green fluorescence it imparts to alkaline solutions—a colour visible even at dilutions of 1:50,000,000. It is used as a dye to colour liquids in analytic instruments, in cosmetics, and as a water tracer or marker. Halogenated derivatives made from fluorescein also include eosin and erythrosin.
Q19) Explain the Preparation and Uses of malachite.
A19) The leuco form of malachite green was first prepared by Hermann Fischer in 1877 by condensing benzaldehyde and dimethylaniline in the molecular ratio 1:2 in the presence of sulfuric acid.

Uses
Malachite green is traditionally used as a dye. Kilotonnes of MG and related triarylmethane dyes are produced annually for this purpose.
MG is active against the oomycete Saprolegnia, which infects fish eggs in commercial aquaculture, MG has been used to treat Saprolegnia and is used as an antibacterial. It is a very popular treatment against Ichthyophthirius multifiliis in freshwater aquaria. The principal metabolite, LMG, is found in fish treated with malachite green, and this finding is the basis of controversy and government regulation. See also Antimicrobials in aquaculture.
Q20) Explain the preparation of Crystal Violet.
A20) Crystal violet can be made in a number of different ways. Kern and Caro created the initial process, which required reacting dimethylaniline with phosgene to produce 4,4′-bis(dimethylamino)benzophenone (Michler's ketone) as an intermediate. In the presence of phosphorus oxychloride and hydrochloric acid, this was then reacted with further dimethylaniline.
The dye can also be prepared by the condensation of formaldehyde and dimethylaniline to give a leuco dye:
CH2O + 3 C6H5N(CH3)2 → CH(C6H4N(CH3)2)3 + H2O
Second, this colourless compound is oxidized to the coloured cationic form: (A typical oxidizing agent is manganese dioxide).
CH(C6H4N(CH3)2)3 + HCl + 1⁄2 O2 → [C(C6H4N(CH3)2)3]Cl + H2O