Unit - 2
Wave Packet
Q1) Write Short note on Superposition of Waves.
A1)
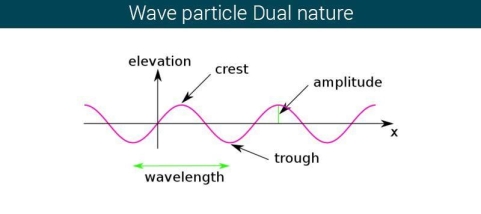
The majority of waves do not appear to be straightforward. They resemble the waves depicted in Figure rather than the ordinary water wave discussed in Waves. (Simple waves have a sinusoidal shape because they are formed by a simple harmonic oscillation.) Complex waves are more intriguing, even beautiful, but they appear to be intimidating. The majority of waves appear complex because they are made up of multiple simple waves that have been added together. Fortunately, the principles for adding waves are straightforward.

When two or more waves arrive at the same location at the same time, they superimpose on each other. When waves collide, the disturbances of the waves are superimposed, a phenomenon known as superposition. Each disruption is associated with a force, which adds up. If the disturbances all follow the same path, the final wave is simply the sum of the individual waves' disturbances—that is, their amplitudes add. Figures show superposition in two different situations, both of which yield straightforward results.
Figure depicts two similar waves arriving at the same time and in phase. The crests and troughs of the two waves are perfectly aligned. The result of this superposition is pure constructive interference. Pure constructive interference generates a wave with double the amplitude of the component waves but the same wavelength since the disturbances add up.
Figure illustrates two identical waves arriving out of phase—that is, with their crests and troughs precisely aligned—producing pure destructive interference. The resulting amplitude for pure destructive interference is 0 since the disturbances are in the opposite direction for this superposition—the waves entirely cancel.
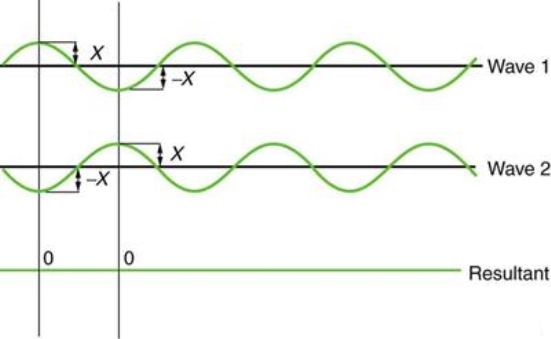
Figure: When two identical waves collide, the result is zero amplitude, or total cancellation.
Q2) Explain Standing Waves.
A2)
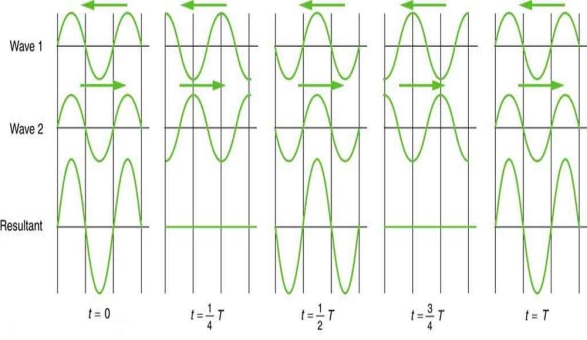
Waves don't always appear to move; instead, they only vibrate in situ. Unmoving waves, for example, can be seen on the surface of a glass of milk in the refrigerator. The refrigerator motor's vibrations create waves on the milk that oscillate up and down but do not appear to travel over the surface. These waves are created by the superposition of two or more moving waves, such as the two identical waves flowing in opposite directions seen in Figure 5. The waves pass through each other, adding to the disruptions as they travel. When the amplitude and wavelength of two waves are the same, they alternate between constructive and destructive interference. The outcome is known as a standing wave because it resembles a wave that is stationary. Standing waves can be seen on the surface of a glass of milk. Other standing waves can be found in guitar strings and organ pipes. The two waves that generate standing waves in the glass of milk could be caused by reflections from the glass's side.
A detailed examination of earthquakes reveals evidence of resonance, standing waves, and constructive and destructive interference situations. A building may be vibrated for several seconds at a frequency that matches the natural frequency of vibration of the building, resulting in a resonance that causes one building to collapse while surrounding buildings remain intact. Buildings of a specific height are frequently destroyed, whereas taller structures remain standing. The height of the building corresponds to the conditions for creating a standing wave at that height. At specific spots along the Earth's surface, constructive interference occurs as seismic waves reflect off denser rocks. Areas near to the epicentre are frequently unaffected, whereas areas further away are.
Q3) What is Group Velocity?
A3)
Something more than a basic harmonic wave is required to transmit information. The superposition of several such waves of differing frequencies, on the other hand, can produce a "envelope" wave and a carrier wave within the envelope. The envelope has the ability to convey data. The superposition of two harmonic waves with very near frequencies (1 2) and the same amplitude is a simple example. The motion's equations are as follows:
u(x, t) = A0 . Cos (ω1 t – k1 x) + A0 . Cos (ω2t – k2 x)
= 2 A0 . Cos ( 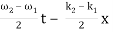 ) × cos (
) × cos ( 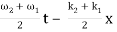 )
)
= u1 × u2
Figure depicts the plot of such a wave.
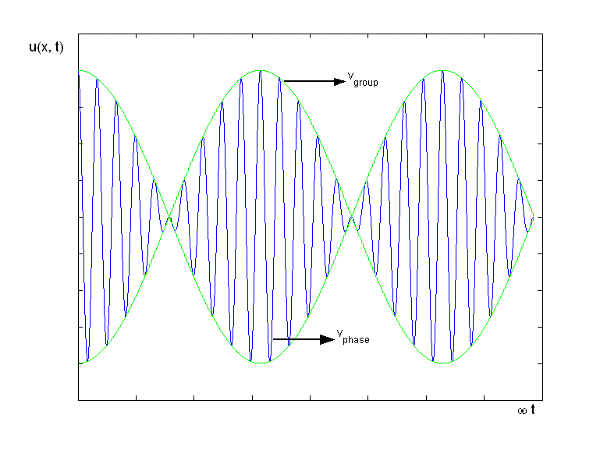
Figure: Group Velocity
u1 controls the envelope (the green line), which moves at the group velocity. The phase velocity of the carrier wave (the blue line) is provided by u2. The wave packet travels at the same speed as the rest of the group. It is the envelope that contains the data. The velocity of a group and the velocity of a phase are not always the same. The velocity of a group is given by,

Rayleigh's formula links phase and group velocity together.
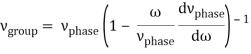
Group velocity equals phase velocity if the derivative term is zero. There is no dispersion in this scenario. When the different phase velocities of the envelope components cause the wave packet to "spread out" over time, this is called dispersion. The wave packet's (or envelope's) components separate to the point that they can no longer combine to complete the envelope.
Q4) Explain Wave-Particle Puzzle in Details.
A4)
De Broglie's explanation of the Bohr atom quantization principles, together with Davisson and Germer's unintentional discovery of electron diffraction scattering, offer a compelling case for the electron's wave nature. Even so, the electron can act like a particle at times. An electron has a certain mass and charge, may move slowly, and can pass through a variety of devices, including a gun and a screen.
So, what's the connection between the wave and particle perspectives? Both were always present, according to De Broglie. He dubbed the wave a pilot wave, and he believed it steered the particle's motion. Unfortunately, the point of view leads to inconsistencies. The common current view is that the relative likelihood of detecting the particle at any position is determined by the strength of the wave (measured by the square of its amplitude).
The probability of finding a quantum (photon) at any point is proportional to the energy density of the field at that point, which is the square of the electric field vector plus the square of the magnetic field vector. This interpretation, first proposed by Max Born in 1926, is analogous to the relationship between the electromagnetic field and quanta—the probability of finding a quantum (photon) at any point is proportional to the energy density of the field at that point, which is the square of the electric field vector plus the square of The de Broglie wave function associated with the electron is written in standard notation as (x,t). The relative probability of finding the electron in a narrow period of length x near location x at time t is thus |(x,t)|2dx. (For the sake of simplicity, we limit the electron's movement to one dimension.) The generalisation is easy to understand.)
Q5) What is Gaussian wave packets?
A5)
We write a free wave packet as a linear combination of plane waves

Where the momentum amplitudes absorb the time evolution of each plane wave, given by (k)=k2/2m (k,t). As a Gaussian, we chose the amplitude for the various plane waves.
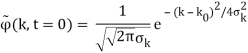
The momentum distribution |φ~(k,t)|2| is a Gaussian of width σk and mean k0 for all t.
At t=0, where the momentum amplitudes are real, the probability density of the resulting wave packet at is a Gaussian of width σx=1/2σk, i.e., it is as localized as is allowed by the Heisenberg principle (σx=1/2). The probability distribution stays Gaussian for all t. As the momentum amplitudes become complex, its width 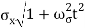 increases with a characteristic time 1/ωσ=2m
increases with a characteristic time 1/ωσ=2m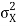 /ℏ, and its center moves with the group velocity vg=ℏk0/m.
/ℏ, and its center moves with the group velocity vg=ℏk0/m.
The genuinely quantum mechanical aspect is the behaviour at t=0: We can't locate the particle any farther than the uncertainty principle allows. It is a direct result of the wave-description resulting directly from the Fourier transform's features. For long periods of time, the behaviour is consistent with that of an ensemble of classical particles with a Gaussian momentum distribution: A particle with momentum k will be at x(k,t)=kt/m at time tt. We get the probability density of discovering a classical particle by solving for k and inserting it into the momentum distribution.
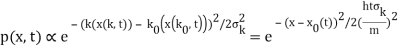
Which is a Gaussian of width σxωσt centered at
x0(t) = hk0t/m
The probability distribution for the wave packets has been plotted so far. We'll need a mechanism to visualise complex functions if we wish to draw the wave function. We could just plot the real and imagined parts of it. However, demonstrating its modulus (the square of which yields the probability density) and phase is more enlightening. It's simple to plot the modulus. We use colours to depict the phases, starting with red for phase zero, progressing through the rainbow colours as the phase progresses, and finally returning to red for phase two. The representation of a plane wave is shown below as an example. 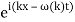
The propagating free Gaussian wave packet's wave function is given by
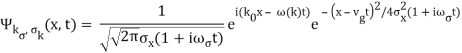
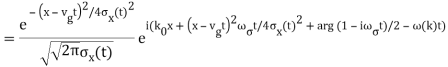
The phase of a Gaussian wave packet with a small momentum distribution is virtually identical to a plane wave with momentum k0.
The phase no longer has a well-defined wave length as the momentum dispersion widens. The phase has wave length 2/k0 around the maximum at x(t)=vgt.
Q6) Give Two Examples of Localized Wave Packets
A6)
Lets now try two examples of a wave packet localized in k and properly normalized at t=0.
- A “square” packet: A(k) =
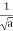 for k0 – a/2 < k < k0 + a/2 and 0 elsewhere.
for k0 – a/2 < k < k0 + a/2 and 0 elsewhere. - A Gaussian packet: A(k) = (2a/π)1/4 e-α (k – k0)2.
These are both localized in momentum about p= hk0.
Check the normalization of (1).

Check the normalization of (2) using the result for a definite integral of a Gaussian
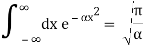

So now we take the Fourier Transform of (1) right here.

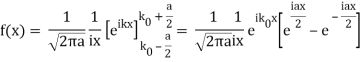

Note that 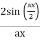 is equal to 1 at x = 0 and that it decreases from there. If you square this, it should remind you of a single slit diffraction pattern! In fact, the single slit gives us a square localization in position space and the F.T. Is this
is equal to 1 at x = 0 and that it decreases from there. If you square this, it should remind you of a single slit diffraction pattern! In fact, the single slit gives us a square localization in position space and the F.T. Is this 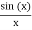 function.
function.
The Fourier Transform of a Gaussian wave packet
A(k) = 
f(x) = 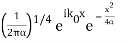
Also a Gaussian. We will show later that a Gaussian is the best one can do to localize a particle in position and momentum at the same time.
In both of these cases of f(x) (transformed from a normalized A(k) localized in momentum space) we see
- A coefficient which correctly normalizes the state to 1,
- eik0x - a wave corresponding to momentum hk0,
- And a packet function which is localized in x.
We've found states that represent a single free particle, which was our goal. We can have states that are localised in both position and momentum space, as we can see. We did this by creating wave packets, which are superpositions of definite-momentum states. While the wave packets are localised, they do have some width. x and in p.
Q7) What is Wave-Particle Duality?
A7)
We've all heard about the nature of light and the various personalities it exhibits. Some of the features include interference, reflection, refraction, and diffraction. Particle-Wave The concept of duality aids us in comprehending the particle and wave aspects of light.
Based on the premise that light and all other electromagnetic radiation can be classified as either particles or waves, physicist Louis De Broglie proposed in 1923 that the same duality must apply to matter. Any particle of matter with momentum (p) has a corresponding wavelength (), according to him.
De Broglie Wavelength formula is given by λ= h/p
Where, h is the Planck constant
For a particle of momentum mv, the wavelength is given by λ=h/mv
This equation is true for photons as well.
De Broglie realised early in the twentieth century that both models describe the same phenomena. Particles can be described as waves, and waves can be described as particles. Light has a wave-like character as revealed by diffraction and interference events, but it also has a particle-like character as revealed by the photoelectric effect, absorption, and emission by atoms. Although electrons have mass, they may be diffracted in the same way that caverns can. Nature shows particle duality and ambiguity in ways that science does not. While the interpretation of this wave-particle duality is still debated, many physicists today embrace the Bohr complement-yarn principle. Both models are necessary for a thorough description of nature, yet they are mutually exclusive. e.
Energy is emitted as quanta, which are small packets of energy, according to Planck's Hypothesis of Quantum Theory. He claims that the energy emitted is proportional to the frequency of the emitted light, a concept known as Wave-Particle Duality. The quantum energy is connected to the frequency by the equation E = hv, according to Planck's hypothesis.
One of the simplest methods to demonstrate the duality between a particle and a wave is to see a light. Because light is akin to waves, it can diffract, refract, interface, and so on.
De Broglie's Theory benefited greatly from Albert Einstein's photoelectric effect theory, which proved that particles and waves may collide. Light can also be viewed as a photon, which is a small particle. Electrons are released when light is viewed on certain objects. To remove one electron from an object's surface, a specific amount of energy is required. When a photon with more energy than an electron collides with a material, an electron is emitted.
Q8) Write Short note on duality of photons.
A8)
“Light is a particle as well as a wave.” To have a better understanding of this concept, we conducted an experiment.
Young's Interference Experiment, often known as the Double-slit Interference Experiment, is a type of experiment. This experiment used technology to identify individual light particles to see if interference fringes appeared even when the light was severely attenuated to the point where there was just one particle. The experiment's findings confirmed that one photon had an interference fringe.
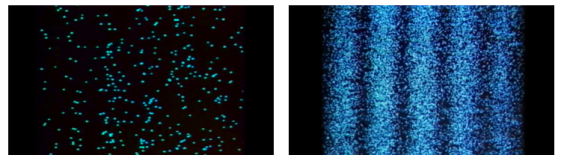
When light is detected that has been weakened to an extreme brightness limit and projected on a screen, it behaves like a particle, as shown on the left. When the number of particles detected rises, however, an interference fringe emerges, as shown on the right. As can be seen, light behaves similarly to a wave.
There was no interference fringe when one of the two slits in the experiment was closed, allowing only one photon particle to pass through the other slit. This proved that in the double-slit interference experiment, one photon particle travelled through both slits at the same time and interfered with itself.
These experiments show that while a photon was detected as having the properties of a particle, interference appeared like that of a wave while simultaneously passing through the double-slit, revealing that the photon has the dual properties of a particle and a wave.
Q9) What is Complementarity?
A9)
Complementarity is a concept in quantum mechanics that Niels Bohr considered to be a necessary property of the theory. According to the complementarity principle, objects have a set of complementary features that cannot all be viewed or measured at the same time. Position and momentum are an example of such a pair. One of quantum mechanics' foundational truths, according to Bohr, is that setting up an experiment to measure one of a pair of quantities, such as the position of an electron, excludes the possibility of measuring the other, despite the fact that understanding both experiments is required to characterise the object under study. The behaviour of atomic and subatomic things, according to Bohr, cannot be divorced from the measuring devices that form the framework in which they behave. As a result, there is no "one picture" that unifies the data gained in these various experimental situations, and only the "totality of the phenomena" as a whole can provide a complete description.
The failure of the operators that represent the observable quantities being measured to commute expresses complementarity mathematically:

Incompatible observables are observables that correlate to non-commutative operators. There can't be a comprehensive set of shared eigenstates for incompatible observables. It's worth noting that there may be some simultaneous eigenstates of () A and () B, but not enough to form a complete basis. The canonical commutation relation is defined as follows:

This suggests that this holds true for both position and momentum. Similarly, any two of the spin observables specified by the Pauli matrices have an equivalent relationship; measurements of spin along perpendicular axes are complimentary. Using mutually unbiased bases, which give complementary observables defined on finite-dimensional Hilbert spaces, this has been applied to discrete observables with more than two possible outcomes.
The principle of complementarity, or dialecticism, is a second key component of quantum physics. Is light a wave or a particle? Complementarity is defined as the recognition that though particle and wave behaviour are mutually exclusive, both are required for a complete explanation of all occurrences.
Q10) What is Light Diffraction?
A10)
It is commonly known that light has the ability to diffract around things in its path, resulting in a pattern of interference that is unique to the object. In reality, this is how holography works (the interference pattern is created by allowing the diffracted light to interfere with the original beam so that the hologram can be viewed by shining the original beam on the image). The Young double slit experiment is a basic demonstration of light diffraction (Figure).
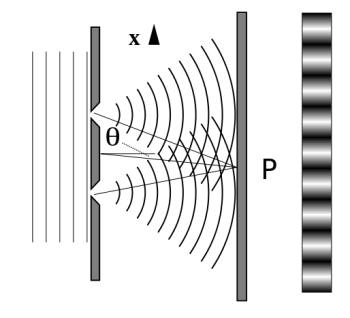
Figure: Experiment with a young double slit. A plane of light waves illuminates two apertures.
Water waves (shown as waves in a plane parallel to the double slit device) are used in this experiment to see what occurs when they hit the slits. Each slit then acts as a point source for spherical waves, which interfere with one another, resulting in the light and dark fringes seen on the right.
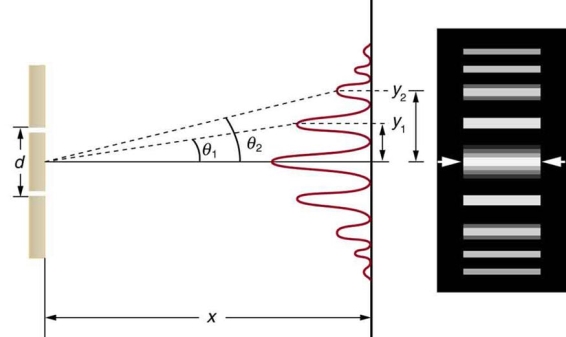 Figure: Interference through a double slit.
Figure: Interference through a double slit.
Q11) Explain Davisson-Germer Experiment.
A11)
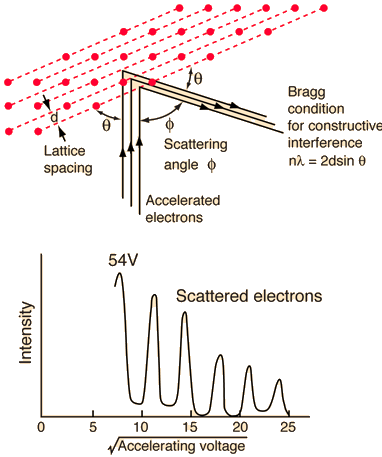
The Davisson-Germer experiment revealed the electron's wave character, validating deBroglie's earlier hypothesis. It was a big step forward in the development of quantum mechanics because it put wave-particle duality on a firm experimental footing. The Bragg diffraction law had previously been applied to x-ray diffraction, but this was the first time it had been applied to particle waves.
Davisson and Germer devised and constructed a vacuum apparatus for determining the energy of electrons scattered from a metal surface. A voltage was used to accelerate electrons from a hot filament and allow them to strike the surface of nickel metal.
The electron beam was focused on a nickel target that could be rotated to observe the dispersed electrons' angular dependency. Their electron detector (known as a Faraday box) was positioned on an arc and could be rotated to observe electrons from various angles. They were taken aback when they discovered that the intensity of the dispersed electron beam had a peak at some angles. The Bragg law could be used to calculate the lattice spacing in the nickel crystal based on this peak, which showed wave behaviour for the electrons.
With rising accelerating voltage, the experimental data above, replicated above Davisson's article, reveals recurrent maxima of scattered electron intensity. This information was gathered at a certain dispersion angle. The relationship is calculated using the Bragg law, the deBroglie wavelength expression, and the kinetic energy of the accelerated electrons.

Electron Bragg deBroglie Acceleration wavelength law relationship through voltage V
An accelerating voltage of 54 volts produced a distinct peak at a scattering angle of 50° in the historical data. The Bragg law's angle theta for that scattering angle is 65°, and the computed lattice spacing for that angle is 0.092 nm. The wavelength as a function of voltage relationship is empirically determined for that lattice spacing and scattering angle.
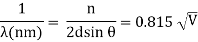
Trying this relationship for n=1,2,3 gives values for the square root of voltage 7.36, 14.7 and 22, which appear to agree with the first, third and fifth peaks above. What then accounts for the second, fourth, and sixth peaks? They could have come from a different set of crystal planes. Those peaks fall into the 2,3,4 sequence, implying that the series' first peak would have been around 5.85.
Q12) Explain Illustration of the principle through experiment of gamma ray microscope and electron.
A12)
Are the uncertainty relations revealed by Heisenberg in 1927 simply the outcome of the equations utilised, or are they embedded in every measurement? Heisenberg used a thought experiment because he felt that all scientific concepts must be defined using actual or possible experimental observations.
Heisenberg imagined a microscope that achieves extremely fine resolution by illuminating with high-energy gamma rays. Although such a microscope does not exist at the moment, it may theoretically be built. Heisenberg envisaged seeing an electron and measuring its position using this microscope. He discovered that the electron's position and momentum obeyed the mathematically established uncertainty relation. The experiment had significant problems, which Bohr pointed out, but after they were fixed, the demonstration was completely compelling.
A free electron rests just beneath the centre of the microscope's lens in the revised version of the thought experiment (see the picture above). From the electron, the circular lens forms a cone with an angle of 2A. Gamma rays—high intensity light with the smallest wavelength—then illuminate the electron from the left. These have the maximum resolution because, according to a wave optics principle, the microscope can resolve (that is, "see" or distinguish) objects to a size of Dx, which is related to and to the gamma ray wavelength L by the expression:
Dx = L / (2sinA)
A gamma ray impacting an electron, on the other hand, provides it a kick in quantum mechanics, where a light wave can act like a particle. The electron is driven to the right when light is diffracted by the electron into the microscope lens. The gamma ray must be dispersed into any angle within the cone of angle 2A in order to be detected by the microscope. The gamma ray, like a particle, carries momentum in quantum physics. The wavelength is related to the total momentum p by the formula
p = h / L, where h is Planck's constant.
The total momentum in the x direction would be the sum of the electron's momentum p'x in the x direction and the gamma ray's momentum in the x direction in the extreme case of diffraction of the gamma ray to the right edge of the lens:
p'x + (h sinA ) / L', where L' is the wavelength of the deflected gamma ray.
In the other extreme, the observed gamma ray recoils backward, just hitting the left edge of the lens. The overall momentum in the x direction in this example is:
p''x - (h sinA ) / L''.
Because momentum is never lost, the final x momentum in each scenario must equal the beginning x momentum (it is conserved). As a result, the final x momenta are identical:
p'x + (h sinA ) / L' = p''x - (h sinA ) / L''
If A is tiny, the wavelengths are close to being the same, L' L" L. So there you have it.
p''x - p'x = Dpx ~ 2h sinA / L
We have a reciprocal link between the minimal uncertainty in the observed position, Dx, of the electron along the x axis and the uncertainty in its momentum, Dpx, in the x direction because Dx = L / (2 sinA ).
Dpx ~ h / Dx or Dpx Dx ~ h.
The "more than" indication can be used if there is more than the bare minimum of uncertainty.
Q13) What is Single slit Experiment?
A13)
Interference, refraction, reflection, and diffraction are all examples of processes that occur as light travels through air. Diffraction of light occurs when it comes into contact with an obstruction.
The wavefront on the other side of the opening mimics the wave when light flows through a small opening that is comparable in size to the wavelength of the light. Please explain light diffraction and single slit diffraction, which occurs when light passes through a single slit.
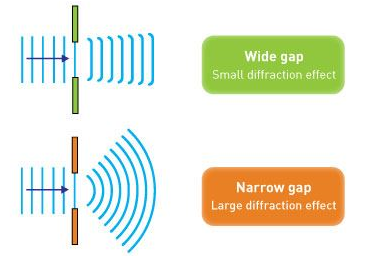
When light passes through a single slit with a width (w) on the order of the wavelength of the light, we can witness single slit diffraction. The screen's diffraction pattern will be at a distance L >> w from the slit. The intensity is proportional to the angle.
We may examine the bending phenomena of light, or diffraction, in the single-slit diffraction experiment, which causes light from a coherent source to interfere with itself and generate a distinct pattern on the screen termed the diffraction pattern. When the sources are tiny enough to be comparable in size to the wavelength of light, diffraction occurs. This impact can be seen in the diagram below. The spreading out of huge apertures is minor and almost undetectable.
Q14) Write Short note Electron Diffraction.
A14)
Electrons, according to classical physics, should behave like particles, travelling in straight lines and not bending in flight until touched on by an external agent, such as a magnetic field. If we fire a beam of electrons through a double slit onto a detector in this scenario, we should obtain two bands of "hits," similar to what you'd get if you shot a machine gun through the side of a home with two windows - two regions of bullet-marked wall inside, while the remainder would be intact.
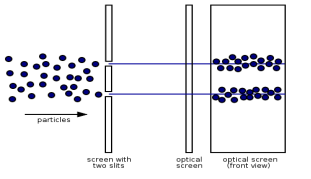
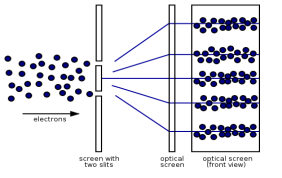
Figure: (left) classical model of electrons. (right) wave property of electrons.
However, if the slits are narrow and close enough together, we can see the electrons diffracting through the slits and interfering with one another, exactly like waves. This indicates that electrons, like photons, exhibit wave-particle duality, which is consistent with de Broglie's concept. They must have wavelength and frequency attributes in this scenario. The attributes can be deduced from the electrons' behaviour as they travel through our diffraction grating.
This was a watershed moment in the history of quantum physics. The Davisson–Germer experiment established the wave-nature of matter, completing the notion of wave-particle duality, much as the photoelectric effect had done for light. This concept was crucial to physicists since it suggested that not only could any particle display wave properties, but that wave equations could be used to describe processes in matter if the de Broglie wavelength was utilised.
Q15) Explain Time Development of a Gaussian Wave Packet.
A15)
So far, we've just done our Fourier Transforms at and examined the results at t = 0. We'll now reintroduce time to the wave function and examine the wave packet at a later time. We'll see that photons and non-relativistic electrons behave very differently.
Assume we start with our Gaussian (minimum uncertainty) wave packet A(k) =  at t = 0. We can do the Fourier Transform to position space, including the time dependence.
at t = 0. We can do the Fourier Transform to position space, including the time dependence.
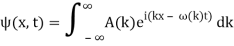
We state directly that is reliant on. This simply indicates that the energy of our free particle is determined by its motion. In the case of a photon, E = pc, so ℏ ω = ℏ kc, and hence ω = kc. For an non-relativistic electron, E = 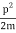 , so ℏ ω =
, so ℏ ω = 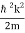 , and hence ω =
, and hence ω =  .
.
To cover the general case, lets expand ω(k) around the center of the wave packet in k-space.
ω(k) = ω(k0) + 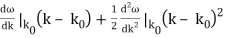
We make some educated guesses about the outcome and give the coefficients names.
ω(k) = ω0 + vg (k – k0) + β (k – k0)2
For the photon, vg = c and β=0. For the NR electron, vg = 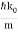 and β =
and β = 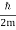 .
.
Performing the Fourier Transform, we get
ψ(x, t) = 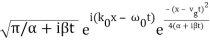
| ψ(x, t)|2 = 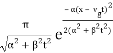
We can see that the photon will travel at the speed of light and that the wave will travel at the same speed.
Packet will not disperse, because β = 0.
The wave packet for the NR electron moves at the correct group velocity., vg = p/m, but the wave packet spreads with time. The RMS width is σ = 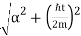
A wave packet naturally spreads because it contains waves of different momenta and hence different velocities. Wave packets that are very localized in space spread rapidly.
Q16) Write Short note on Quantum Harmonic Oscillator.
A16)
One drawback of this traditional formulation is that it is not universal. We can't use it to represent diatomic molecular vibrations, for example, because quantum effects are important. Using the classical statement to limit discussion of a "spring" constant between the atoms is a first step toward a quantum formulation. The potential energy function can be stated in a more general sense this way.
U(x) = ½ m ω2 x2
When this expression is used with the time-independent Schrdinger equation, the result is
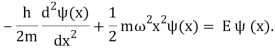
To solve (Figure), we need the wave functions to be symmetric around x=0 (the bottom of the potential well) and normalizable in order to discover the permitted energies E and their corresponding wave functions (x). When integrated throughout the complete range of x from to+, these requirements ensure that the probability density |(x)2 | must be finite. A more advanced course in quantum mechanics will cover how to solve (Figure); here, we will simply mention the results. The permitted energies are as follows:
En = ( n + ½ ) ℏω = 2n+1/2 ℏω, n = 0, 1, 2, 3, . . .
These energies (stationary states or states of defined energy) correlate to wave functions.
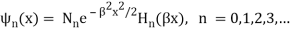
Where β = 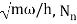 ,
, 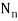 is the normalization constant, and Hn(y) is a polynomial of degree n called a Hermite polynomial. The first four Hermite polynomials are
is the normalization constant, and Hn(y) is a polynomial of degree n called a Hermite polynomial. The first four Hermite polynomials are
H0 (y) = 1
H1 (y) = 2y
H2 (y) = 4y2 – 2
H3 (y) = 8y3 – 12y
In this section, we'll look at a few sample wave functions (Figure). As the primary number grows larger, the solutions alternate between even and odd functions around the axis.
Q17) What is the Estimate Hydrogen Ground State Energy?
A17)
The fundamentally uncertainty principle explains why the hydrogen atom (and other atoms) is so big. If the electron were to be contained in a smaller space,∆p would increase, causing p to increase on average. The energy would rise rather than fall.
The uncertainty principle can be used to calculate the minimal energy for hydrogen. This computation is not perfect, but it is more accurate than the Bohr model. The notion is that the radius must be greater than the positional spread, and the momentum must be greater than the momentum spread.
r ≥ ∆x
p ≥ ∆p = ℏ/2r
In terms of the dimensionless fine structure constant, this is our formula for potential energy. α.
V(r) = 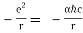
Let us calculate the energy.
E = 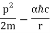
And include the uncertainty principle's influence.
Pr = ℏ
E = 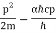
E = 
Differentiate in terms of p To get the minimum, set equal to zero.
DE/dp = p/m – 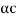 = 0
= 0
p = 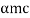

It's worth noting that potential energy is just (-2) times kinetic energy (as we expect from the Virial Theorem). The energy formula for the ground state is valid.
We can also make an educated guess at the radius.

Hydrogen has no (orbital) angular momentum in its ground state. It is not travelling in a circular orbit as predicted by Bohr. The probability distribution of the electron is spread out over around 1. The energy would rise if it wasn't spread out.
Q18) Write Short note on Uncertainty.
A18)
With difficulties of wave/particle duality, classical physics was on shaky ground, but the discovery of the uncertainty principle took them completely off surprise.
The uncertainty principle, also known as the Heisenberg Uncertainty Principle or Indeterminacy Principle, was proposed by German physicist Werner Heisenberg in 1927, and states that an object's position and velocity cannot be measured precisely at the same time, even in theory. In fact, in nature, the concepts of absolute position and exact velocity have no relevance.
This principle is not obvious from everyday life. Because the uncertainties suggested by this concept for common objects are sufficiently small to be noticed, it is simple to measure both the position and velocity of, say, a car. The full rule states that the product of position and velocity uncertainties is equal to or larger than a minuscule physical amount, or constant (about 10-34 joule-second, the value of the quantity h (where h is Planck's constant)). Only for atoms and subatomic particles with extremely small masses can the product of uncertainty become important.
Any attempt to precisely measure the velocity of a subatomic particle, such as an electron, will cause it to move around in an unpredictable manner, rendering a simultaneous measurement of its position useless. This finding originates from the intimate relationship in nature between particles and waves in the world of subatomic dimensions, and has nothing to do with shortcomings in the measurement devices, procedure, or observer.
Every particle has a wave connected with it, and every particle behaves in a wavelike manner. The particle is most likely to be located where the wave's undulations are the most pronounced or powerful. However, the more powerful the associated wave's undulations get, the more ill-defined the wavelength becomes, which defines the particle's momentum. As a result, a strictly localised wave has an indeterminate wavelength, while its associated particle has a fixed position but no fixed velocity. A particle wave with a well-defined wavelength, on the other hand, is widely dispersed; the associated particle, albeit having a very accurate velocity, may be found practically anywhere. A very precise measurement of one observable entails a significant amount of uncertainty in the measurement of the other.
The uncertainty principle can also be represented in terms of the momentum and position of a particle. A particle's momentum is equal to the product of its mass and velocity. As a result, the product of a particle's momentum and position uncertainty equals h/(2) or greater. The principle holds for other (conjugate) pairings of observables, such as energy and time: the product of the uncertainty in an energy measurement and the uncertainty in the time interval during which the measurement is taken equals h/(2) or greater. The uncertainty in the quantity of energy radiated and the uncertainty in the lifetime of the unstable system as it transitions to a more stable state are the same for an unstable atom or nucleus.
Q19) Explain Principle of Superposition of Waves.
A19)
Consider two waves travelling in opposite directions along the same stretched string, as shown in the diagram above. At any given time, we can see images of waveforms in the string. The algebraic total of the displacements owing to each wave is found to be the net displacement of every element of the string at any given time.
Let's imagine two waves are travelling alone, and any element of these two waves' displacements can be represented as y1(x, t) and y2(x, t) (x, t). The resultant displacement can be written as y when these two waves collide (x,t).
y (x, t) = y1(x, t) + y2(x, t) = y3(x, t) = y4(x, t) = y5(x, t) = y (x, t)
We can sum the overlapped waves algebraically to generate a consequent wave using the principle of superposition. Let us say that the wave functions of moving waves are as follows:
y1 = f1(x–vt),
y2 = f2(x–vt)
……….
yn = fn (x–vt)
Then the wave function describing the disturbance in the medium can be described as
y = f1(x – vt)+ f2(x – vt)+ …+ fn(x – vt)
Or, y=∑ i=1 to n = fi (x−vt)
Let us consider a wave travelling along a stretched string given by, y1(x, t) = A sin (kx – ωt) and another wave, shifted from the first by a phase φ, given as y2(x, t) = A sin (kx – ωt + φ)
From the equations we can see that both the waves have the same angular frequency, same angular wave number k, hence the same wavelength and the same amplitude A.
Now, applying the superposition principle, the resultant wave is the algebraic sum of the two constituent waves and has displacement y(x, t) = A sin (kx – ωt) + A sin (kx – ωt + φ)
The above equation can be written as,
y(x, t) = 2A cos (ϕ/2). Sin (kx − ωt + ϕ/2)
The resulting wave is a sinusoidal wave travelling in the positive X direction, with a phase angle half that of the component waves and an amplitude equal to [2cos/2] times the original waves' amplitudes.
Q20) Write Short note on Localizing an Electron.
A20)
To solve this question, consider the wave function corresponding to an electron moving in a vacuum tube, for example. The electron exits the cathode, travels through the vacuum, and collides with a grid's anode. It is travelling through the vacuum at a moment in this process, and the wave function must be nonzero across some volume, but zero in places the electron has not yet reached and zero in places it has definitely gone.
The electron, on the other hand, has a precise momentum if it possesses a precise energy, such as fifty electron volts. This necessitates the existence of a definite wavelength for the wave. However, the only wave with an exact wavelength is (x,t)=Asin(kxt).
Where k=2/ and =2f are constants. The issue is that this plane sine wave stretches to infinity in both spatial directions, therefore it can't represent a particle with a non-zero wave function in a finite region of space.
As a result, we must superpose waves of different wavelengths to represent a confined particle. Using the trigonometric addition formula, superpose two waves with somewhat varying wavelengths to demonstrate the principle:
Sin((kk)x(+)t)=2 sin((k+k)x(+)t)=2 sin((k+k)x(+)t)=2 sin((k+k)x(+)t)=2 sin((k+k)x(+)t)=2 sin((
Sin(kxt)cos((k)x()t sin(kxt)cos((k)x()t sin(k)x()t sin(k)x()t sin
The phenomena of beats between waves of similar frequency is represented by this formula. The first term, sin(kxt), oscillates at a frequency that is the average of the two. The slowly fluctuating second term, which oscillates once over a spatial range of order /k, modulates it. This is the distance at which waves that were in phase at the start turn fully out of phase. Of course, if the distance between the waves is increased by order /k, the waves will re-synchronize.
That is, combining two close frequencies creates a series of packets, or beats, from the continuous wave. A single packet is required to describe a single electron travelling through space. This can be accomplished by superposing waves with a continuous distribution of wavelengths, or wave numbers in the order of k, for example. The waves will be out of phase after a distance of order /k, but because they have so many distinct wavelengths, they will never be able to get back in phase. (All of this is adequately treated in Fourier transform theory.) We're just attempting to make it plausible right now.)
Unit - 2
Wave Packet
Q1) Write Short note on Superposition of Waves.
A1)
The majority of waves do not appear to be straightforward. They resemble the waves depicted in Figure rather than the ordinary water wave discussed in Waves. (Simple waves have a sinusoidal shape because they are formed by a simple harmonic oscillation.) Complex waves are more intriguing, even beautiful, but they appear to be intimidating. The majority of waves appear complex because they are made up of multiple simple waves that have been added together. Fortunately, the principles for adding waves are straightforward.

When two or more waves arrive at the same location at the same time, they superimpose on each other. When waves collide, the disturbances of the waves are superimposed, a phenomenon known as superposition. Each disruption is associated with a force, which adds up. If the disturbances all follow the same path, the final wave is simply the sum of the individual waves' disturbances—that is, their amplitudes add. Figures show superposition in two different situations, both of which yield straightforward results.
Figure depicts two similar waves arriving at the same time and in phase. The crests and troughs of the two waves are perfectly aligned. The result of this superposition is pure constructive interference. Pure constructive interference generates a wave with double the amplitude of the component waves but the same wavelength since the disturbances add up.
Figure illustrates two identical waves arriving out of phase—that is, with their crests and troughs precisely aligned—producing pure destructive interference. The resulting amplitude for pure destructive interference is 0 since the disturbances are in the opposite direction for this superposition—the waves entirely cancel.
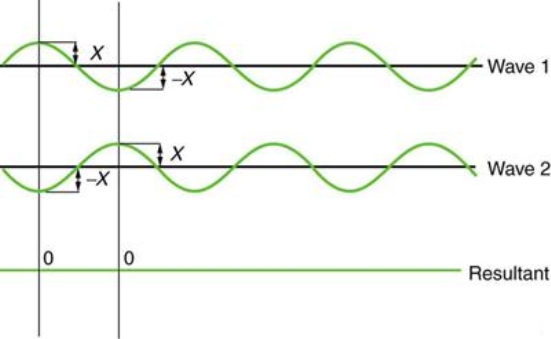
Figure: When two identical waves collide, the result is zero amplitude, or total cancellation.
Q2) Explain Standing Waves.
A2)
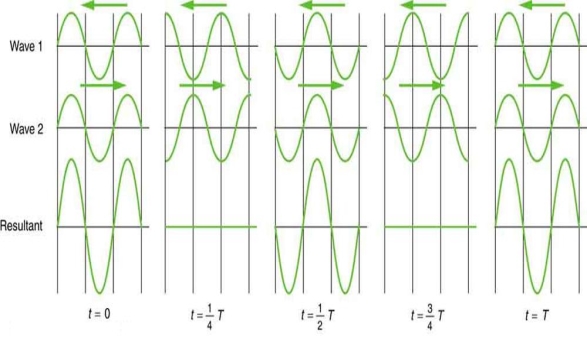
Waves don't always appear to move; instead, they only vibrate in situ. Unmoving waves, for example, can be seen on the surface of a glass of milk in the refrigerator. The refrigerator motor's vibrations create waves on the milk that oscillate up and down but do not appear to travel over the surface. These waves are created by the superposition of two or more moving waves, such as the two identical waves flowing in opposite directions seen in Figure 5. The waves pass through each other, adding to the disruptions as they travel. When the amplitude and wavelength of two waves are the same, they alternate between constructive and destructive interference. The outcome is known as a standing wave because it resembles a wave that is stationary. Standing waves can be seen on the surface of a glass of milk. Other standing waves can be found in guitar strings and organ pipes. The two waves that generate standing waves in the glass of milk could be caused by reflections from the glass's side.
A detailed examination of earthquakes reveals evidence of resonance, standing waves, and constructive and destructive interference situations. A building may be vibrated for several seconds at a frequency that matches the natural frequency of vibration of the building, resulting in a resonance that causes one building to collapse while surrounding buildings remain intact. Buildings of a specific height are frequently destroyed, whereas taller structures remain standing. The height of the building corresponds to the conditions for creating a standing wave at that height. At specific spots along the Earth's surface, constructive interference occurs as seismic waves reflect off denser rocks. Areas near to the epicentre are frequently unaffected, whereas areas further away are.
Q3) What is Group Velocity?
A3)
Something more than a basic harmonic wave is required to transmit information. The superposition of several such waves of differing frequencies, on the other hand, can produce a "envelope" wave and a carrier wave within the envelope. The envelope has the ability to convey data. The superposition of two harmonic waves with very near frequencies (1 2) and the same amplitude is a simple example. The motion's equations are as follows:
u(x, t) = A0 . Cos (ω1 t – k1 x) + A0 . Cos (ω2t – k2 x)
= 2 A0 . Cos (  ) × cos (
) × cos ( 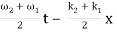 )
)
= u1 × u2
Figure depicts the plot of such a wave.
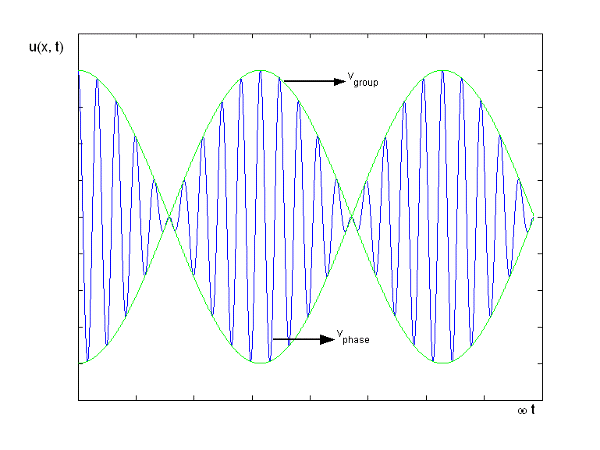
Figure: Group Velocity
u1 controls the envelope (the green line), which moves at the group velocity. The phase velocity of the carrier wave (the blue line) is provided by u2. The wave packet travels at the same speed as the rest of the group. It is the envelope that contains the data. The velocity of a group and the velocity of a phase are not always the same. The velocity of a group is given by,

Rayleigh's formula links phase and group velocity together.
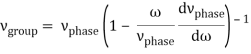
Group velocity equals phase velocity if the derivative term is zero. There is no dispersion in this scenario. When the different phase velocities of the envelope components cause the wave packet to "spread out" over time, this is called dispersion. The wave packet's (or envelope's) components separate to the point that they can no longer combine to complete the envelope.
Q4) Explain Wave-Particle Puzzle in Details.
A4)
De Broglie's explanation of the Bohr atom quantization principles, together with Davisson and Germer's unintentional discovery of electron diffraction scattering, offer a compelling case for the electron's wave nature. Even so, the electron can act like a particle at times. An electron has a certain mass and charge, may move slowly, and can pass through a variety of devices, including a gun and a screen.
So, what's the connection between the wave and particle perspectives? Both were always present, according to De Broglie. He dubbed the wave a pilot wave, and he believed it steered the particle's motion. Unfortunately, the point of view leads to inconsistencies. The common current view is that the relative likelihood of detecting the particle at any position is determined by the strength of the wave (measured by the square of its amplitude).
The probability of finding a quantum (photon) at any point is proportional to the energy density of the field at that point, which is the square of the electric field vector plus the square of the magnetic field vector. This interpretation, first proposed by Max Born in 1926, is analogous to the relationship between the electromagnetic field and quanta—the probability of finding a quantum (photon) at any point is proportional to the energy density of the field at that point, which is the square of the electric field vector plus the square of The de Broglie wave function associated with the electron is written in standard notation as (x,t). The relative probability of finding the electron in a narrow period of length x near location x at time t is thus |(x,t)|2dx. (For the sake of simplicity, we limit the electron's movement to one dimension.) The generalisation is easy to understand.)
Q5) What is Gaussian wave packets?
A5)
We write a free wave packet as a linear combination of plane waves

Where the momentum amplitudes absorb the time evolution of each plane wave, given by (k)=k2/2m (k,t). As a Gaussian, we chose the amplitude for the various plane waves.
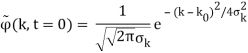
The momentum distribution |φ~(k,t)|2| is a Gaussian of width σk and mean k0 for all t.
At t=0, where the momentum amplitudes are real, the probability density of the resulting wave packet at is a Gaussian of width σx=1/2σk, i.e., it is as localized as is allowed by the Heisenberg principle (σx=1/2). The probability distribution stays Gaussian for all t. As the momentum amplitudes become complex, its width 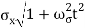 increases with a characteristic time 1/ωσ=2m
increases with a characteristic time 1/ωσ=2m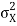 /ℏ, and its center moves with the group velocity vg=ℏk0/m.
/ℏ, and its center moves with the group velocity vg=ℏk0/m.
The genuinely quantum mechanical aspect is the behaviour at t=0: We can't locate the particle any farther than the uncertainty principle allows. It is a direct result of the wave-description resulting directly from the Fourier transform's features. For long periods of time, the behaviour is consistent with that of an ensemble of classical particles with a Gaussian momentum distribution: A particle with momentum k will be at x(k,t)=kt/m at time tt. We get the probability density of discovering a classical particle by solving for k and inserting it into the momentum distribution.
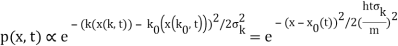
Which is a Gaussian of width σxωσt centered at
x0(t) = hk0t/m
The probability distribution for the wave packets has been plotted so far. We'll need a mechanism to visualise complex functions if we wish to draw the wave function. We could just plot the real and imagined parts of it. However, demonstrating its modulus (the square of which yields the probability density) and phase is more enlightening. It's simple to plot the modulus. We use colours to depict the phases, starting with red for phase zero, progressing through the rainbow colours as the phase progresses, and finally returning to red for phase two. The representation of a plane wave is shown below as an example. 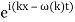
The propagating free Gaussian wave packet's wave function is given by
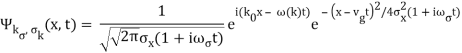
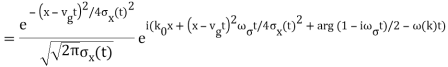
The phase of a Gaussian wave packet with a small momentum distribution is virtually identical to a plane wave with momentum k0.
The phase no longer has a well-defined wave length as the momentum dispersion widens. The phase has wave length 2/k0 around the maximum at x(t)=vgt.
Q6) Give Two Examples of Localized Wave Packets
A6)
Lets now try two examples of a wave packet localized in k and properly normalized at t=0.
- A “square” packet: A(k) =
 for k0 – a/2 < k < k0 + a/2 and 0 elsewhere.
for k0 – a/2 < k < k0 + a/2 and 0 elsewhere. - A Gaussian packet: A(k) = (2a/π)1/4 e-α (k – k0)2.
These are both localized in momentum about p= hk0.
Check the normalization of (1).
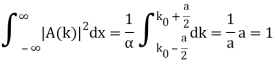
Check the normalization of (2) using the result for a definite integral of a Gaussian


So now we take the Fourier Transform of (1) right here.
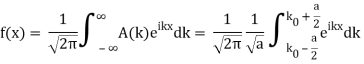
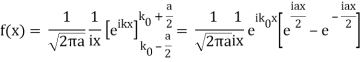

Note that 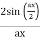 is equal to 1 at x = 0 and that it decreases from there. If you square this, it should remind you of a single slit diffraction pattern! In fact, the single slit gives us a square localization in position space and the F.T. Is this
is equal to 1 at x = 0 and that it decreases from there. If you square this, it should remind you of a single slit diffraction pattern! In fact, the single slit gives us a square localization in position space and the F.T. Is this 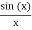 function.
function.
The Fourier Transform of a Gaussian wave packet
A(k) = 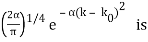
f(x) = 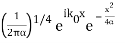
Also a Gaussian. We will show later that a Gaussian is the best one can do to localize a particle in position and momentum at the same time.
In both of these cases of f(x) (transformed from a normalized A(k) localized in momentum space) we see
- A coefficient which correctly normalizes the state to 1,
- eik0x - a wave corresponding to momentum hk0,
- And a packet function which is localized in x.
We've found states that represent a single free particle, which was our goal. We can have states that are localised in both position and momentum space, as we can see. We did this by creating wave packets, which are superpositions of definite-momentum states. While the wave packets are localised, they do have some width. x and in p.
Q7) What is Wave-Particle Duality?
A7)
We've all heard about the nature of light and the various personalities it exhibits. Some of the features include interference, reflection, refraction, and diffraction. Particle-Wave The concept of duality aids us in comprehending the particle and wave aspects of light.
Based on the premise that light and all other electromagnetic radiation can be classified as either particles or waves, physicist Louis De Broglie proposed in 1923 that the same duality must apply to matter. Any particle of matter with momentum (p) has a corresponding wavelength (), according to him.
De Broglie Wavelength formula is given by λ= h/p
Where, h is the Planck constant
For a particle of momentum mv, the wavelength is given by λ=h/mv
This equation is true for photons as well.
De Broglie realised early in the twentieth century that both models describe the same phenomena. Particles can be described as waves, and waves can be described as particles. Light has a wave-like character as revealed by diffraction and interference events, but it also has a particle-like character as revealed by the photoelectric effect, absorption, and emission by atoms. Although electrons have mass, they may be diffracted in the same way that caverns can. Nature shows particle duality and ambiguity in ways that science does not. While the interpretation of this wave-particle duality is still debated, many physicists today embrace the Bohr complement-yarn principle. Both models are necessary for a thorough description of nature, yet they are mutually exclusive. e.
Energy is emitted as quanta, which are small packets of energy, according to Planck's Hypothesis of Quantum Theory. He claims that the energy emitted is proportional to the frequency of the emitted light, a concept known as Wave-Particle Duality. The quantum energy is connected to the frequency by the equation E = hv, according to Planck's hypothesis.
One of the simplest methods to demonstrate the duality between a particle and a wave is to see a light. Because light is akin to waves, it can diffract, refract, interface, and so on.
De Broglie's Theory benefited greatly from Albert Einstein's photoelectric effect theory, which proved that particles and waves may collide. Light can also be viewed as a photon, which is a small particle. Electrons are released when light is viewed on certain objects. To remove one electron from an object's surface, a specific amount of energy is required. When a photon with more energy than an electron collides with a material, an electron is emitted.
Q8) Write Short note on duality of photons.
A8)
“Light is a particle as well as a wave.” To have a better understanding of this concept, we conducted an experiment.
Young's Interference Experiment, often known as the Double-slit Interference Experiment, is a type of experiment. This experiment used technology to identify individual light particles to see if interference fringes appeared even when the light was severely attenuated to the point where there was just one particle. The experiment's findings confirmed that one photon had an interference fringe.
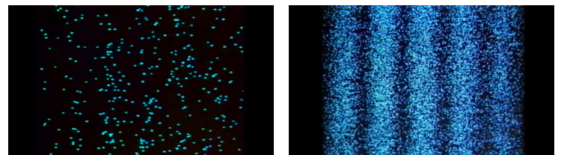
When light is detected that has been weakened to an extreme brightness limit and projected on a screen, it behaves like a particle, as shown on the left. When the number of particles detected rises, however, an interference fringe emerges, as shown on the right. As can be seen, light behaves similarly to a wave.
There was no interference fringe when one of the two slits in the experiment was closed, allowing only one photon particle to pass through the other slit. This proved that in the double-slit interference experiment, one photon particle travelled through both slits at the same time and interfered with itself.
These experiments show that while a photon was detected as having the properties of a particle, interference appeared like that of a wave while simultaneously passing through the double-slit, revealing that the photon has the dual properties of a particle and a wave.
Q9) What is Complementarity?
A9)
Complementarity is a concept in quantum mechanics that Niels Bohr considered to be a necessary property of the theory. According to the complementarity principle, objects have a set of complementary features that cannot all be viewed or measured at the same time. Position and momentum are an example of such a pair. One of quantum mechanics' foundational truths, according to Bohr, is that setting up an experiment to measure one of a pair of quantities, such as the position of an electron, excludes the possibility of measuring the other, despite the fact that understanding both experiments is required to characterise the object under study. The behaviour of atomic and subatomic things, according to Bohr, cannot be divorced from the measuring devices that form the framework in which they behave. As a result, there is no "one picture" that unifies the data gained in these various experimental situations, and only the "totality of the phenomena" as a whole can provide a complete description.
The failure of the operators that represent the observable quantities being measured to commute expresses complementarity mathematically:
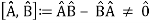
Incompatible observables are observables that correlate to non-commutative operators. There can't be a comprehensive set of shared eigenstates for incompatible observables. It's worth noting that there may be some simultaneous eigenstates of () A and () B, but not enough to form a complete basis. The canonical commutation relation is defined as follows:
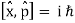
This suggests that this holds true for both position and momentum. Similarly, any two of the spin observables specified by the Pauli matrices have an equivalent relationship; measurements of spin along perpendicular axes are complimentary. Using mutually unbiased bases, which give complementary observables defined on finite-dimensional Hilbert spaces, this has been applied to discrete observables with more than two possible outcomes.
The principle of complementarity, or dialecticism, is a second key component of quantum physics. Is light a wave or a particle? Complementarity is defined as the recognition that though particle and wave behaviour are mutually exclusive, both are required for a complete explanation of all occurrences.
Q10) What is Light Diffraction?
A10)
It is commonly known that light has the ability to diffract around things in its path, resulting in a pattern of interference that is unique to the object. In reality, this is how holography works (the interference pattern is created by allowing the diffracted light to interfere with the original beam so that the hologram can be viewed by shining the original beam on the image). The Young double slit experiment is a basic demonstration of light diffraction (Figure).
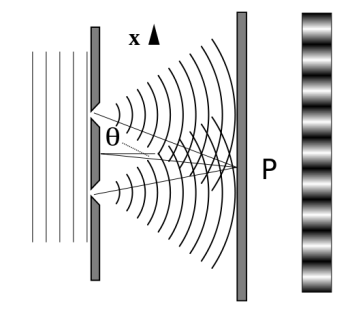
Figure: Experiment with a young double slit. A plane of light waves illuminates two apertures.
Water waves (shown as waves in a plane parallel to the double slit device) are used in this experiment to see what occurs when they hit the slits. Each slit then acts as a point source for spherical waves, which interfere with one another, resulting in the light and dark fringes seen on the right.
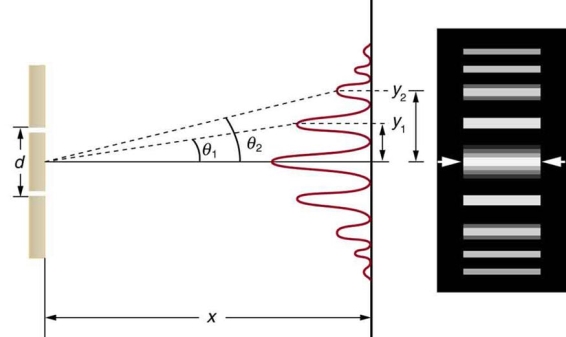 Figure: Interference through a double slit.
Figure: Interference through a double slit.
Q11) Explain Davisson-Germer Experiment.
A11)
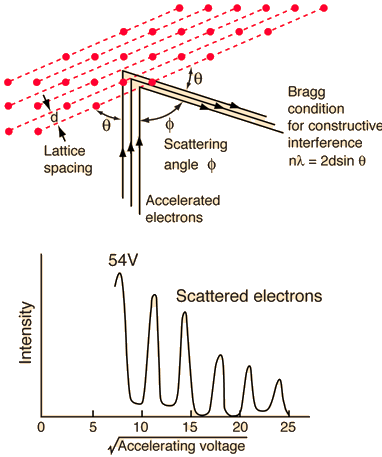
The Davisson-Germer experiment revealed the electron's wave character, validating deBroglie's earlier hypothesis. It was a big step forward in the development of quantum mechanics because it put wave-particle duality on a firm experimental footing. The Bragg diffraction law had previously been applied to x-ray diffraction, but this was the first time it had been applied to particle waves.
Davisson and Germer devised and constructed a vacuum apparatus for determining the energy of electrons scattered from a metal surface. A voltage was used to accelerate electrons from a hot filament and allow them to strike the surface of nickel metal.
The electron beam was focused on a nickel target that could be rotated to observe the dispersed electrons' angular dependency. Their electron detector (known as a Faraday box) was positioned on an arc and could be rotated to observe electrons from various angles. They were taken aback when they discovered that the intensity of the dispersed electron beam had a peak at some angles. The Bragg law could be used to calculate the lattice spacing in the nickel crystal based on this peak, which showed wave behaviour for the electrons.
With rising accelerating voltage, the experimental data above, replicated above Davisson's article, reveals recurrent maxima of scattered electron intensity. This information was gathered at a certain dispersion angle. The relationship is calculated using the Bragg law, the deBroglie wavelength expression, and the kinetic energy of the accelerated electrons.
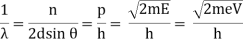
Electron Bragg deBroglie Acceleration wavelength law relationship through voltage V
An accelerating voltage of 54 volts produced a distinct peak at a scattering angle of 50° in the historical data. The Bragg law's angle theta for that scattering angle is 65°, and the computed lattice spacing for that angle is 0.092 nm. The wavelength as a function of voltage relationship is empirically determined for that lattice spacing and scattering angle.
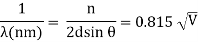
Trying this relationship for n=1,2,3 gives values for the square root of voltage 7.36, 14.7 and 22, which appear to agree with the first, third and fifth peaks above. What then accounts for the second, fourth, and sixth peaks? They could have come from a different set of crystal planes. Those peaks fall into the 2,3,4 sequence, implying that the series' first peak would have been around 5.85.
Q12) Explain Illustration of the principle through experiment of gamma ray microscope and electron.
A12)
Are the uncertainty relations revealed by Heisenberg in 1927 simply the outcome of the equations utilised, or are they embedded in every measurement? Heisenberg used a thought experiment because he felt that all scientific concepts must be defined using actual or possible experimental observations.
Heisenberg imagined a microscope that achieves extremely fine resolution by illuminating with high-energy gamma rays. Although such a microscope does not exist at the moment, it may theoretically be built. Heisenberg envisaged seeing an electron and measuring its position using this microscope. He discovered that the electron's position and momentum obeyed the mathematically established uncertainty relation. The experiment had significant problems, which Bohr pointed out, but after they were fixed, the demonstration was completely compelling.
A free electron rests just beneath the centre of the microscope's lens in the revised version of the thought experiment (see the picture above). From the electron, the circular lens forms a cone with an angle of 2A. Gamma rays—high intensity light with the smallest wavelength—then illuminate the electron from the left. These have the maximum resolution because, according to a wave optics principle, the microscope can resolve (that is, "see" or distinguish) objects to a size of Dx, which is related to and to the gamma ray wavelength L by the expression:
Dx = L / (2sinA)
A gamma ray impacting an electron, on the other hand, provides it a kick in quantum mechanics, where a light wave can act like a particle. The electron is driven to the right when light is diffracted by the electron into the microscope lens. The gamma ray must be dispersed into any angle within the cone of angle 2A in order to be detected by the microscope. The gamma ray, like a particle, carries momentum in quantum physics. The wavelength is related to the total momentum p by the formula
p = h / L, where h is Planck's constant.
The total momentum in the x direction would be the sum of the electron's momentum p'x in the x direction and the gamma ray's momentum in the x direction in the extreme case of diffraction of the gamma ray to the right edge of the lens:
p'x + (h sinA ) / L', where L' is the wavelength of the deflected gamma ray.
In the other extreme, the observed gamma ray recoils backward, just hitting the left edge of the lens. The overall momentum in the x direction in this example is:
p''x - (h sinA ) / L''.
Because momentum is never lost, the final x momentum in each scenario must equal the beginning x momentum (it is conserved). As a result, the final x momenta are identical:
p'x + (h sinA ) / L' = p''x - (h sinA ) / L''
If A is tiny, the wavelengths are close to being the same, L' L" L. So there you have it.
p''x - p'x = Dpx ~ 2h sinA / L
We have a reciprocal link between the minimal uncertainty in the observed position, Dx, of the electron along the x axis and the uncertainty in its momentum, Dpx, in the x direction because Dx = L / (2 sinA ).
Dpx ~ h / Dx or Dpx Dx ~ h.
The "more than" indication can be used if there is more than the bare minimum of uncertainty.
Q13) What is Single slit Experiment?
A13)
Interference, refraction, reflection, and diffraction are all examples of processes that occur as light travels through air. Diffraction of light occurs when it comes into contact with an obstruction.
The wavefront on the other side of the opening mimics the wave when light flows through a small opening that is comparable in size to the wavelength of the light. Please explain light diffraction and single slit diffraction, which occurs when light passes through a single slit.
When light passes through a single slit with a width (w) on the order of the wavelength of the light, we can witness single slit diffraction. The screen's diffraction pattern will be at a distance L >> w from the slit. The intensity is proportional to the angle.
We may examine the bending phenomena of light, or diffraction, in the single-slit diffraction experiment, which causes light from a coherent source to interfere with itself and generate a distinct pattern on the screen termed the diffraction pattern. When the sources are tiny enough to be comparable in size to the wavelength of light, diffraction occurs. This impact can be seen in the diagram below. The spreading out of huge apertures is minor and almost undetectable.
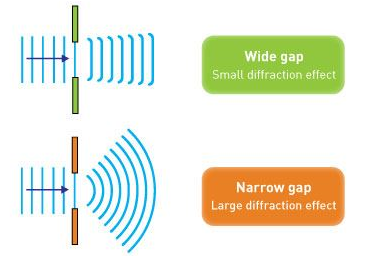
Q14) Write Short note Electron Diffraction.
A14)
Electrons, according to classical physics, should behave like particles, travelling in straight lines and not bending in flight until touched on by an external agent, such as a magnetic field. If we fire a beam of electrons through a double slit onto a detector in this scenario, we should obtain two bands of "hits," similar to what you'd get if you shot a machine gun through the side of a home with two windows - two regions of bullet-marked wall inside, while the remainder would be intact.
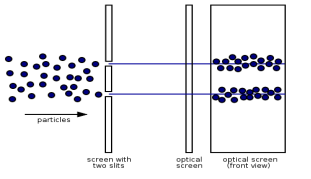
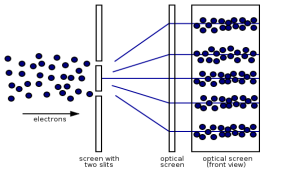
Figure: (left) classical model of electrons. (right) wave property of electrons.
However, if the slits are narrow and close enough together, we can see the electrons diffracting through the slits and interfering with one another, exactly like waves. This indicates that electrons, like photons, exhibit wave-particle duality, which is consistent with de Broglie's concept. They must have wavelength and frequency attributes in this scenario. The attributes can be deduced from the electrons' behaviour as they travel through our diffraction grating.
This was a watershed moment in the history of quantum physics. The Davisson–Germer experiment established the wave-nature of matter, completing the notion of wave-particle duality, much as the photoelectric effect had done for light. This concept was crucial to physicists since it suggested that not only could any particle display wave properties, but that wave equations could be used to describe processes in matter if the de Broglie wavelength was utilised.
Q15) Explain Time Development of a Gaussian Wave Packet.
A15)
So far, we've just done our Fourier Transforms at and examined the results at t = 0. We'll now reintroduce time to the wave function and examine the wave packet at a later time. We'll see that photons and non-relativistic electrons behave very differently.
Assume we start with our Gaussian (minimum uncertainty) wave packet A(k) = 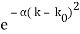 at t = 0. We can do the Fourier Transform to position space, including the time dependence.
at t = 0. We can do the Fourier Transform to position space, including the time dependence.
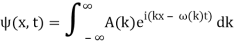
We state directly that is reliant on. This simply indicates that the energy of our free particle is determined by its motion. In the case of a photon, E = pc, so ℏ ω = ℏ kc, and hence ω = kc. For an non-relativistic electron, E = 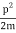 , so ℏ ω =
, so ℏ ω = 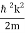 , and hence ω =
, and hence ω = 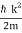 .
.
To cover the general case, lets expand ω(k) around the center of the wave packet in k-space.
ω(k) = ω(k0) + 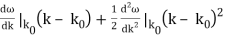
We make some educated guesses about the outcome and give the coefficients names.
ω(k) = ω0 + vg (k – k0) + β (k – k0)2
For the photon, vg = c and β=0. For the NR electron, vg = 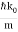 and β =
and β = 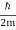 .
.
Performing the Fourier Transform, we get
ψ(x, t) = 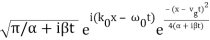
| ψ(x, t)|2 = 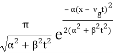
We can see that the photon will travel at the speed of light and that the wave will travel at the same speed.
Packet will not disperse, because β = 0.
The wave packet for the NR electron moves at the correct group velocity., vg = p/m, but the wave packet spreads with time. The RMS width is σ = 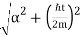
A wave packet naturally spreads because it contains waves of different momenta and hence different velocities. Wave packets that are very localized in space spread rapidly.
Q16) Write Short note on Quantum Harmonic Oscillator.
A16)
One drawback of this traditional formulation is that it is not universal. We can't use it to represent diatomic molecular vibrations, for example, because quantum effects are important. Using the classical statement to limit discussion of a "spring" constant between the atoms is a first step toward a quantum formulation. The potential energy function can be stated in a more general sense this way.
U(x) = ½ m ω2 x2
When this expression is used with the time-independent Schrdinger equation, the result is
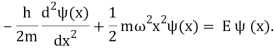
To solve (Figure), we need the wave functions to be symmetric around x=0 (the bottom of the potential well) and normalizable in order to discover the permitted energies E and their corresponding wave functions (x). When integrated throughout the complete range of x from to+, these requirements ensure that the probability density |(x)2 | must be finite. A more advanced course in quantum mechanics will cover how to solve (Figure); here, we will simply mention the results. The permitted energies are as follows:
En = ( n + ½ ) ℏω = 2n+1/2 ℏω, n = 0, 1, 2, 3, . . .
These energies (stationary states or states of defined energy) correlate to wave functions.
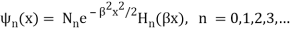
Where β =  ,
,  is the normalization constant, and Hn(y) is a polynomial of degree n called a Hermite polynomial. The first four Hermite polynomials are
is the normalization constant, and Hn(y) is a polynomial of degree n called a Hermite polynomial. The first four Hermite polynomials are
H0 (y) = 1
H1 (y) = 2y
H2 (y) = 4y2 – 2
H3 (y) = 8y3 – 12y
In this section, we'll look at a few sample wave functions (Figure). As the primary number grows larger, the solutions alternate between even and odd functions around the axis.
Q17) What is the Estimate Hydrogen Ground State Energy?
A17)
The fundamentally uncertainty principle explains why the hydrogen atom (and other atoms) is so big. If the electron were to be contained in a smaller space,∆p would increase, causing p to increase on average. The energy would rise rather than fall.
The uncertainty principle can be used to calculate the minimal energy for hydrogen. This computation is not perfect, but it is more accurate than the Bohr model. The notion is that the radius must be greater than the positional spread, and the momentum must be greater than the momentum spread.
r ≥ ∆x
p ≥ ∆p = ℏ/2r
In terms of the dimensionless fine structure constant, this is our formula for potential energy. α.
V(r) = 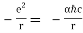
Let us calculate the energy.
E = 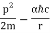
And include the uncertainty principle's influence.
Pr = ℏ
E = 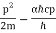
E = 
Differentiate in terms of p To get the minimum, set equal to zero.
DE/dp = p/m – 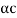 = 0
= 0
p = 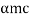
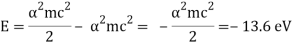
It's worth noting that potential energy is just (-2) times kinetic energy (as we expect from the Virial Theorem). The energy formula for the ground state is valid.
We can also make an educated guess at the radius.
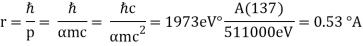
Hydrogen has no (orbital) angular momentum in its ground state. It is not travelling in a circular orbit as predicted by Bohr. The probability distribution of the electron is spread out over around 1. The energy would rise if it wasn't spread out.
Q18) Write Short note on Uncertainty.
A18)
With difficulties of wave/particle duality, classical physics was on shaky ground, but the discovery of the uncertainty principle took them completely off surprise.
The uncertainty principle, also known as the Heisenberg Uncertainty Principle or Indeterminacy Principle, was proposed by German physicist Werner Heisenberg in 1927, and states that an object's position and velocity cannot be measured precisely at the same time, even in theory. In fact, in nature, the concepts of absolute position and exact velocity have no relevance.
This principle is not obvious from everyday life. Because the uncertainties suggested by this concept for common objects are sufficiently small to be noticed, it is simple to measure both the position and velocity of, say, a car. The full rule states that the product of position and velocity uncertainties is equal to or larger than a minuscule physical amount, or constant (about 10-34 joule-second, the value of the quantity h (where h is Planck's constant)). Only for atoms and subatomic particles with extremely small masses can the product of uncertainty become important.
Any attempt to precisely measure the velocity of a subatomic particle, such as an electron, will cause it to move around in an unpredictable manner, rendering a simultaneous measurement of its position useless. This finding originates from the intimate relationship in nature between particles and waves in the world of subatomic dimensions, and has nothing to do with shortcomings in the measurement devices, procedure, or observer.
Every particle has a wave connected with it, and every particle behaves in a wavelike manner. The particle is most likely to be located where the wave's undulations are the most pronounced or powerful. However, the more powerful the associated wave's undulations get, the more ill-defined the wavelength becomes, which defines the particle's momentum. As a result, a strictly localised wave has an indeterminate wavelength, while its associated particle has a fixed position but no fixed velocity. A particle wave with a well-defined wavelength, on the other hand, is widely dispersed; the associated particle, albeit having a very accurate velocity, may be found practically anywhere. A very precise measurement of one observable entails a significant amount of uncertainty in the measurement of the other.
The uncertainty principle can also be represented in terms of the momentum and position of a particle. A particle's momentum is equal to the product of its mass and velocity. As a result, the product of a particle's momentum and position uncertainty equals h/(2) or greater. The principle holds for other (conjugate) pairings of observables, such as energy and time: the product of the uncertainty in an energy measurement and the uncertainty in the time interval during which the measurement is taken equals h/(2) or greater. The uncertainty in the quantity of energy radiated and the uncertainty in the lifetime of the unstable system as it transitions to a more stable state are the same for an unstable atom or nucleus.
Q19) Explain Principle of Superposition of Waves.
A19)
Consider two waves travelling in opposite directions along the same stretched string, as shown in the diagram above. At any given time, we can see images of waveforms in the string. The algebraic total of the displacements owing to each wave is found to be the net displacement of every element of the string at any given time.
Let's imagine two waves are travelling alone, and any element of these two waves' displacements can be represented as y1(x, t) and y2(x, t) (x, t). The resultant displacement can be written as y when these two waves collide (x,t).
y (x, t) = y1(x, t) + y2(x, t) = y3(x, t) = y4(x, t) = y5(x, t) = y (x, t)
We can sum the overlapped waves algebraically to generate a consequent wave using the principle of superposition. Let us say that the wave functions of moving waves are as follows:
y1 = f1(x–vt),
y2 = f2(x–vt)
……….
yn = fn (x–vt)
Then the wave function describing the disturbance in the medium can be described as
y = f1(x – vt)+ f2(x – vt)+ …+ fn(x – vt)
Or, y=∑ i=1 to n = fi (x−vt)
Let us consider a wave travelling along a stretched string given by, y1(x, t) = A sin (kx – ωt) and another wave, shifted from the first by a phase φ, given as y2(x, t) = A sin (kx – ωt + φ)
From the equations we can see that both the waves have the same angular frequency, same angular wave number k, hence the same wavelength and the same amplitude A.
Now, applying the superposition principle, the resultant wave is the algebraic sum of the two constituent waves and has displacement y(x, t) = A sin (kx – ωt) + A sin (kx – ωt + φ)
The above equation can be written as,
y(x, t) = 2A cos (ϕ/2). Sin (kx − ωt + ϕ/2)
The resulting wave is a sinusoidal wave travelling in the positive X direction, with a phase angle half that of the component waves and an amplitude equal to [2cos/2] times the original waves' amplitudes.
Q20) Write Short note on Localizing an Electron.
A20)
To solve this question, consider the wave function corresponding to an electron moving in a vacuum tube, for example. The electron exits the cathode, travels through the vacuum, and collides with a grid's anode. It is travelling through the vacuum at a moment in this process, and the wave function must be nonzero across some volume, but zero in places the electron has not yet reached and zero in places it has definitely gone.
The electron, on the other hand, has a precise momentum if it possesses a precise energy, such as fifty electron volts. This necessitates the existence of a definite wavelength for the wave. However, the only wave with an exact wavelength is (x,t)=Asin(kxt).
Where k=2/ and =2f are constants. The issue is that this plane sine wave stretches to infinity in both spatial directions, therefore it can't represent a particle with a non-zero wave function in a finite region of space.
As a result, we must superpose waves of different wavelengths to represent a confined particle. Using the trigonometric addition formula, superpose two waves with somewhat varying wavelengths to demonstrate the principle:
Sin((kk)x(+)t)=2 sin((k+k)x(+)t)=2 sin((k+k)x(+)t)=2 sin((k+k)x(+)t)=2 sin((k+k)x(+)t)=2 sin((
Sin(kxt)cos((k)x()t sin(kxt)cos((k)x()t sin(k)x()t sin(k)x()t sin
The phenomena of beats between waves of similar frequency is represented by this formula. The first term, sin(kxt), oscillates at a frequency that is the average of the two. The slowly fluctuating second term, which oscillates once over a spatial range of order /k, modulates it. This is the distance at which waves that were in phase at the start turn fully out of phase. Of course, if the distance between the waves is increased by order /k, the waves will re-synchronize.
That is, combining two close frequencies creates a series of packets, or beats, from the continuous wave. A single packet is required to describe a single electron travelling through space. This can be accomplished by superposing waves with a continuous distribution of wavelengths, or wave numbers in the order of k, for example. The waves will be out of phase after a distance of order /k, but because they have so many distinct wavelengths, they will never be able to get back in phase. (All of this is adequately treated in Fourier transform theory.) We're just attempting to make it plausible right now.)