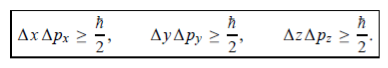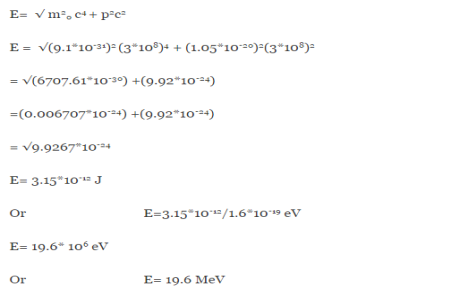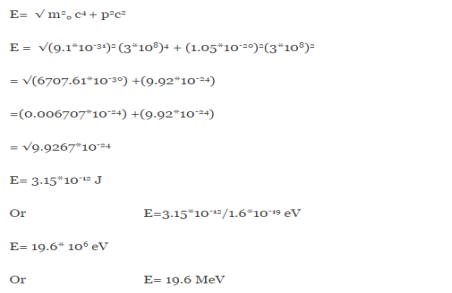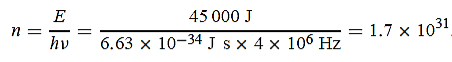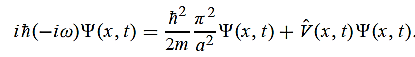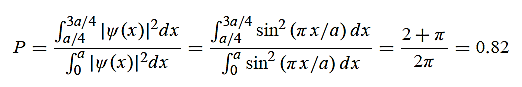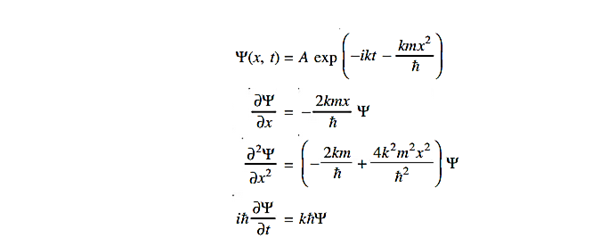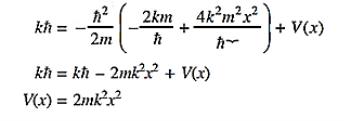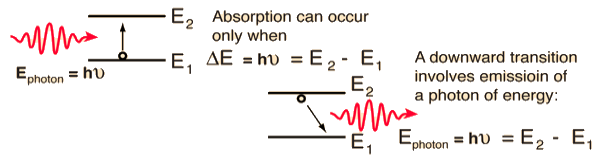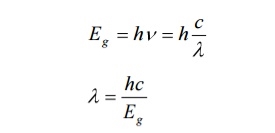behaves as a group of waves(matter waves) whose wavelength λand wave vector
behaves as a group of waves(matter waves) whose wavelength λand wave vector 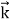 are governed by the speed and mass of the particle. De Broglie proposed that the wave length λ associated with a particle of momentum p is given as where m is the mass of the particle and v its speed. λ =
are governed by the speed and mass of the particle. De Broglie proposed that the wave length λ associated with a particle of momentum p is given as where m is the mass of the particle and v its speed. λ =  =
=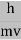 …….(1)
…….(1)  =
=  …….(2) Where ℏ = h/2π. The expression known as the deBroglie relation connects the momentum of a particle with the wavelength and wave vector of the wave corresponding to this particle.The wavelength λ of the matter wave is called de Broglie wavelength. The dual aspect of matter is evident in the de Broglie relation. λ is the attribute of a wave while on the right hand side the momentum p is a typical attribute of a particle. Planck’s constant h relates the two attributes. Equation (1) for a material particle is basically a hypothesis whose validity can be tested only by experiment. However, it is interesting to see that it is satisfied also by a photon. For a photon, as we have seen, p = hν/c. Therefore
…….(2) Where ℏ = h/2π. The expression known as the deBroglie relation connects the momentum of a particle with the wavelength and wave vector of the wave corresponding to this particle.The wavelength λ of the matter wave is called de Broglie wavelength. The dual aspect of matter is evident in the de Broglie relation. λ is the attribute of a wave while on the right hand side the momentum p is a typical attribute of a particle. Planck’s constant h relates the two attributes. Equation (1) for a material particle is basically a hypothesis whose validity can be tested only by experiment. However, it is interesting to see that it is satisfied also by a photon. For a photon, as we have seen, p = hν/c. Therefore =
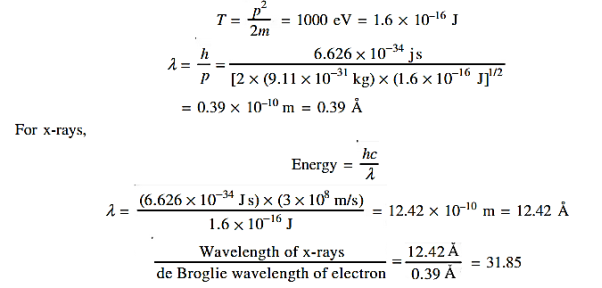
=  = λ Q2) What is the de Broglie wavelength associated with (a) an electron moving with a speed of 5.4×106 m/s, and (b) a ball of mass 150 g travelling at 30.0 m/s?A2) (a)For the electron: Mass m = 9.11×10–31 kg, speed v = 5.4×106 m/s. Then, momentum p = m v = 9.11×10–31 kg × 5.4 × 106 (m/s)p = 4.92 × 10–24 kg m/sde Broglie wavelength, λ = h/p = 6.63 x 10-34Js/ 4.92 × 10–24 kg m/sλ= 0.135 nm(b)For the ball:Mass m’ = 0.150 kg, Speed v ’= 30.0 m/s.Then momentum p’ = m’ v’= 0.150 (kg) × 30.0 (m/s)p’= 4.50 kg m/sde Broglie wavelength λ’ = h/p’ =6.63 x 10-34Js/ 4.50 kg m/s =1.47 ×10–34 mThe de Broglie wavelength of electron is comparable with X-ray wavelengths. However, for the ball it is about 10–19 times the size of the proton, quite beyond experimental measurement.Q3) What is the de Broglie wavelength associated with an electron, accelerated through a potential difference of 100 volts?A3) Accelerating potential V = 100 V. The de Broglie wavelength λ is λ= h /p = 1 227/
= λ Q2) What is the de Broglie wavelength associated with (a) an electron moving with a speed of 5.4×106 m/s, and (b) a ball of mass 150 g travelling at 30.0 m/s?A2) (a)For the electron: Mass m = 9.11×10–31 kg, speed v = 5.4×106 m/s. Then, momentum p = m v = 9.11×10–31 kg × 5.4 × 106 (m/s)p = 4.92 × 10–24 kg m/sde Broglie wavelength, λ = h/p = 6.63 x 10-34Js/ 4.92 × 10–24 kg m/sλ= 0.135 nm(b)For the ball:Mass m’ = 0.150 kg, Speed v ’= 30.0 m/s.Then momentum p’ = m’ v’= 0.150 (kg) × 30.0 (m/s)p’= 4.50 kg m/sde Broglie wavelength λ’ = h/p’ =6.63 x 10-34Js/ 4.50 kg m/s =1.47 ×10–34 mThe de Broglie wavelength of electron is comparable with X-ray wavelengths. However, for the ball it is about 10–19 times the size of the proton, quite beyond experimental measurement.Q3) What is the de Broglie wavelength associated with an electron, accelerated through a potential difference of 100 volts?A3) Accelerating potential V = 100 V. The de Broglie wavelength λ is λ= h /p = 1 227/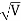 nmλ.1 227/
nmλ.1 227/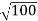 nm = 0.123 nmThe de Broglie wavelength associated with an electron in this case is of the order of x ray wavelengths.Q4) What is Heisenberg’s Uncertainty Principle?A4) According to classical physics, given the initial conditions and the forces acting on a system, the future behaviour (unique path) of this physical system can be determined exactly. That is, if the initial coordinates
nm = 0.123 nmThe de Broglie wavelength associated with an electron in this case is of the order of x ray wavelengths.Q4) What is Heisenberg’s Uncertainty Principle?A4) According to classical physics, given the initial conditions and the forces acting on a system, the future behaviour (unique path) of this physical system can be determined exactly. That is, if the initial coordinates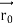 , velocity
, velocity 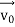 , and all the forces acting on the particle are known, the position
, and all the forces acting on the particle are known, the position 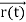 , and velocity
, and velocity  are uniquely determined by means of Newton’s second law. So by Classical physicsit can be easily derived.Does this hold for the microphysical world? Since a particle is represented within the context of quantum mechanics by means of a wave function corresponding to the particle’s wave, and since wave functions cannot be localized, then a microscopic particle is somewhat spread over space and, unlike classical particles, cannot be localized in space. In addition, we have seen in the double-slit experiment that it is impossible to determine the slit that the electron went through without disturbing it. The classical concepts of exact position, exact momentum, and unique path of a particle therefore make no sense at the microscopic scale. This is the essence of Heisenberg’s uncertainty principle. In its original form, Heisenberg’s uncertainty principle states that: If the x-component of the momentum of a particle is measured with an uncertainty ∆px, then its x-position cannot, at the same time, be measured more accurately than ∆x =ℏ/(2∆px). The three-dimensional form of the uncertainty relations for position and momentum can be written as follows:
are uniquely determined by means of Newton’s second law. So by Classical physicsit can be easily derived.Does this hold for the microphysical world? Since a particle is represented within the context of quantum mechanics by means of a wave function corresponding to the particle’s wave, and since wave functions cannot be localized, then a microscopic particle is somewhat spread over space and, unlike classical particles, cannot be localized in space. In addition, we have seen in the double-slit experiment that it is impossible to determine the slit that the electron went through without disturbing it. The classical concepts of exact position, exact momentum, and unique path of a particle therefore make no sense at the microscopic scale. This is the essence of Heisenberg’s uncertainty principle. In its original form, Heisenberg’s uncertainty principle states that: If the x-component of the momentum of a particle is measured with an uncertainty ∆px, then its x-position cannot, at the same time, be measured more accurately than ∆x =ℏ/(2∆px). The three-dimensional form of the uncertainty relations for position and momentum can be written as follows:
|
v = 20m/s,m = 0.5kg,h = 6.626 × 10-34 m2 kg / sΔp =p×1×10−6As we know that,
P = m×v = 0.5×20 = 10kgm/s
Δp = 10×1×10−6Δp = 10-5
Heisenberg Uncertainty principle formula is given as,∆x∆p
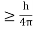 ∆x
∆x  ∆x
∆x 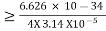 ∆x =0.527 x 10-29 m Q6) Prove non-existence of electrons in the nucleus?A6) One of the applications is to prove that electron cannot exist inside the nucleus. But to prove it, let us assume that electrons exist in the nucleus. As the radius of the nucleus in approximately 10-14m. If electron is to exist inside the nucleus, then uncertainty in the position of the electron is given byAccording to uncertainty principle∆x ∆p =h/2πThus ∆p=h/2π∆xOr ∆p=6.62 x10-34/2 x 3.14 x 10-14Or ∆p=1.05 x 10-20 kg m/ secIf this is p the uncertainty in the momentum of electron then the momentum of electron should be at least of this order that is p=1.05*10-20 kg m/sec.An electron having this much high momentum must have a velocity comparable to the velocity of light. Thus, its energy should be calculated by the following relativistic formulaE =
∆x =0.527 x 10-29 m Q6) Prove non-existence of electrons in the nucleus?A6) One of the applications is to prove that electron cannot exist inside the nucleus. But to prove it, let us assume that electrons exist in the nucleus. As the radius of the nucleus in approximately 10-14m. If electron is to exist inside the nucleus, then uncertainty in the position of the electron is given byAccording to uncertainty principle∆x ∆p =h/2πThus ∆p=h/2π∆xOr ∆p=6.62 x10-34/2 x 3.14 x 10-14Or ∆p=1.05 x 10-20 kg m/ secIf this is p the uncertainty in the momentum of electron then the momentum of electron should be at least of this order that is p=1.05*10-20 kg m/sec.An electron having this much high momentum must have a velocity comparable to the velocity of light. Thus, its energy should be calculated by the following relativistic formulaE = 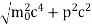
|
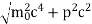
|
 =
= 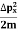 =
= Where mis the mass of the particle.Energy even at O K is given by the above equation. This minimum energy is called the zero-point energy.This implies that even at zero kelvin, the particle is never at rest. If it is so, then ∆pX = 0, which is not possible. [It gives ∆x =∞]3. Existence of proton, neutron and alpha particles within the nucleus.We know that the rest mass of the protons and neutron is of the order of 1.67x 10-27 kg. Hence, the value of momentum 5.27 x 10-21 kg.m/sec from calculation and also the value of v come out to be 3x 105m/sec. The corresponding value of kinetic energy of a neutron or a proton isE=
Where mis the mass of the particle.Energy even at O K is given by the above equation. This minimum energy is called the zero-point energy.This implies that even at zero kelvin, the particle is never at rest. If it is so, then ∆pX = 0, which is not possible. [It gives ∆x =∞]3. Existence of proton, neutron and alpha particles within the nucleus.We know that the rest mass of the protons and neutron is of the order of 1.67x 10-27 kg. Hence, the value of momentum 5.27 x 10-21 kg.m/sec from calculation and also the value of v come out to be 3x 105m/sec. The corresponding value of kinetic energy of a neutron or a proton isE=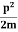 =
= 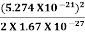 =8.33 x 10-15J =
=8.33 x 10-15J =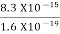 eV
eV 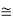 52.05 keVSince the rest mass of the a-particle is nearly four times the proton mass, therefore the alpha particle should have a minimum kinetic energy of one fourth of 52.05 keV, or about 13 keV. Since the energy carried by the protons or neutrons emitted by the nuclei are greater than 52 keV and for a-particle more than 13 keV, these particles can exist in the nuclei. 4. Size of Elementary cell in Phase spaceWe have studied in our previous class that state of a microsystem is defined by six variables – three are due to position and three due to momentum. Hence, a system of N particles needs 6 N variables If ∆ x and ∆px be the uncertainly in position and in momentum measurements then ∆ x ∆ px =
52.05 keVSince the rest mass of the a-particle is nearly four times the proton mass, therefore the alpha particle should have a minimum kinetic energy of one fourth of 52.05 keV, or about 13 keV. Since the energy carried by the protons or neutrons emitted by the nuclei are greater than 52 keV and for a-particle more than 13 keV, these particles can exist in the nuclei. 4. Size of Elementary cell in Phase spaceWe have studied in our previous class that state of a microsystem is defined by six variables – three are due to position and three due to momentum. Hence, a system of N particles needs 6 N variables If ∆ x and ∆px be the uncertainly in position and in momentum measurements then ∆ x ∆ px =  ; Similarly ∆ y ∆ py =
; Similarly ∆ y ∆ py =  , And ∆ z ∆ pz =
, And ∆ z ∆ pz =  , Multiplying these three equations, we get ∆ x ∆ y ∆ z ∆ px ∆ py ∆ pz = (
, Multiplying these three equations, we get ∆ x ∆ y ∆ z ∆ px ∆ py ∆ pz = ( )3 in the units (J3 S3).The above product is called the volume of elementary cell in phase space.So, volume of an elementary cell in phase space
)3 in the units (J3 S3).The above product is called the volume of elementary cell in phase space.So, volume of an elementary cell in phase space 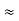 10-101 units, (for quantum statistics) being
10-101 units, (for quantum statistics) being 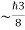 . 5. Accurate limit of frequency of radiation emitted by an atomConsider the radiation emitted from an excited atom. The energy of this atom will decrease when it emits one or more photons of characteristic frequency. The average period between excitation of the atom and the release of energy is about 10-8 seconds. Thus, uncertainly in energy is ∆ E
. 5. Accurate limit of frequency of radiation emitted by an atomConsider the radiation emitted from an excited atom. The energy of this atom will decrease when it emits one or more photons of characteristic frequency. The average period between excitation of the atom and the release of energy is about 10-8 seconds. Thus, uncertainly in energy is ∆ E 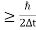 Or ∆ E
Or ∆ E 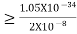 J Or ∆ E
J Or ∆ E 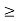 5.3 x 10-27 J Frequency of light is uncertain by ∆ν =
5.3 x 10-27 J Frequency of light is uncertain by ∆ν = 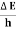 =
= 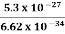 Hz
Hz 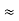 0.8 x 107 HzAs a result, the radiation from an excited atom does not have the noted precise frequency new ν - ∆ν and ν + ∆ν.Q8) Derive the Schrodinger wave equation?A8) Schrodinger wave equation, is the fundamental equation of quantum mechanics, same as the second law of motion is the fundamental equation of classical mechanics. This equation has been derived by Schrodinger in 1925 using the concept of wave function on the basis of de-Broglie wave and plank’s quantum theory.Let us consider a particle of mass m and classically the energy of a particle is the sum of the kinetic and potential energies. We will assume that the potential is a function of only x. So We have E= K+V =
0.8 x 107 HzAs a result, the radiation from an excited atom does not have the noted precise frequency new ν - ∆ν and ν + ∆ν.Q8) Derive the Schrodinger wave equation?A8) Schrodinger wave equation, is the fundamental equation of quantum mechanics, same as the second law of motion is the fundamental equation of classical mechanics. This equation has been derived by Schrodinger in 1925 using the concept of wave function on the basis of de-Broglie wave and plank’s quantum theory.Let us consider a particle of mass m and classically the energy of a particle is the sum of the kinetic and potential energies. We will assume that the potential is a function of only x. So We have E= K+V = mv2+V(x) =
mv2+V(x) =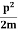 +V(x) ……….. (1) By de Broglie’s relation we know that all particles can be represented as waves with frequency ω and wave number k, and that E= ℏω and p= ℏk. Using this equation (1) for the energy will becomeℏω =
+V(x) ……….. (1) By de Broglie’s relation we know that all particles can be represented as waves with frequency ω and wave number k, and that E= ℏω and p= ℏk. Using this equation (1) for the energy will becomeℏω = 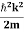 +V(x) ……….. (2) A wave with frequency ω and wave number k can be written as usual asψ(x, t) =Aei(kx−ωt) ……….. (3) the above equation is for one dimensional and for three dimensional we can write it as ψ(r, t) =Aei(k·r−ωt) ……….. (4) But here we will stick to one dimension only.
+V(x) ……….. (2) A wave with frequency ω and wave number k can be written as usual asψ(x, t) =Aei(kx−ωt) ……….. (3) the above equation is for one dimensional and for three dimensional we can write it as ψ(r, t) =Aei(k·r−ωt) ……….. (4) But here we will stick to one dimension only.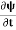 =−iωψ ⇒ ωψ=
=−iωψ ⇒ ωψ=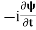 ……….. (5)
……….. (5)  =−k2ψ ⇒ k2ψ = -
=−k2ψ ⇒ k2ψ = - 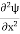 ……….. (6) If we multiply the energy equation in Eq. (2) by ψ, and using(5) and (6) , we obtain ℏ(ωψ) =
……….. (6) If we multiply the energy equation in Eq. (2) by ψ, and using(5) and (6) , we obtain ℏ(ωψ) = 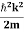 ψ+V(x) ψ ⇒
ψ+V(x) ψ ⇒ 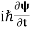 = -
= -  + V(x) ψ……….. (7) This is the time-dependent Schrodinger equation. If we put the x and t in above equation then equation (7) takes the form as given below
+ V(x) ψ……….. (7) This is the time-dependent Schrodinger equation. If we put the x and t in above equation then equation (7) takes the form as given below = -
= -  + V(x) ψ(x,t) ……….. (8) In 3-D, the x dependence turns into dependence on all three coordinates (x, y, z) and the
+ V(x) ψ(x,t) ……….. (8) In 3-D, the x dependence turns into dependence on all three coordinates (x, y, z) and the  term becomes ∇2ψ. The term |ψ(x)|2 gives the probability of finding the particle at position x. Let us again take it as simply a mathematical equation, then it’s just another wave equation. However We already know the solution as we used this function ψ(x, t) =Aei(kx−ωt) to produce Equations (5), (6) and (7) But let’s pretend that we don’t know this, and let’s solve the Schrodinger equation as if we were given to us. As always, we will guess an exponential solution by looking at exponential behaviour in the time coordinate, our guess is ψ(x, t) =e−iωtf(x) putting this into Equation (7) and cancelling the e−iωt yields
term becomes ∇2ψ. The term |ψ(x)|2 gives the probability of finding the particle at position x. Let us again take it as simply a mathematical equation, then it’s just another wave equation. However We already know the solution as we used this function ψ(x, t) =Aei(kx−ωt) to produce Equations (5), (6) and (7) But let’s pretend that we don’t know this, and let’s solve the Schrodinger equation as if we were given to us. As always, we will guess an exponential solution by looking at exponential behaviour in the time coordinate, our guess is ψ(x, t) =e−iωtf(x) putting this into Equation (7) and cancelling the e−iωt yields  = -
= -  + V(x) f(x) ……….. (9) We already know that E=
+ V(x) f(x) ……….. (9) We already know that E=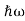 . However ψ(x, t) is general convention to also use the letter ψ to denote the spatial part. So we will now replace f(x) with ψ(x) Eψ = -
. However ψ(x, t) is general convention to also use the letter ψ to denote the spatial part. So we will now replace f(x) with ψ(x) Eψ = - 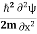 + V(x) ψ ……….. (10) This is called the time-independent Schrodinger equation.The Schrodinger equation also known as Schrodinger’s wave equation is a partial differential equation that describes the dynamics of quantum mechanical systems by the wave function. The trajectory, the positioning, and the energy of these systems can be retrieved by solving the Schrodinger equation.All of the information for a subatomic particle is encoded within a wave function. The wave function will satisfy and can be solved by using the Schrodinger equation. The Schrodinger equation is one of the fundamental axioms that are introduced in undergraduate physics. Q9) The mass of an electron is 9.1×10–31 kg. Its uncertainty in velocity is 5.7×105 m/sec. Calculate uncertainty in its position?A9) Given m= 9×10–31ΔV= 5.7×105 m/sec Δx=?h= 6.6×10–34 Joule-Sec.Δx.Δv ≥h/4πmΔx≥h/4πmΔv≥6.6×10–34/9×3.14×9.1×10–31×5.7×105≥ 0.010×10–8≥ 1×10–10m Q10) The mass of a ball is 0.15 kg & its uncertainty in position to 10–10m. What is the value of uncertainty in its velocity?A10) Given m=0.15 kg. h=6.6×10-34 Joule-Sec.Δx = 10 –10 mΔv=?Δx.Δv ≥h/4πmΔv≥h/4πmΔx≥6.6×10-34/4×3.14×0.15×10–10≥ 3.50×10–24m Q11) The mass of a bullet is 10gm & uncertainty in its velocity is 5.25×10–26 cm/sec. Calculate the uncertainty in its position?A11) m=10 gm. h=6.6×10–27 erg-secΔv= 5.25×10–26 cmΔx=?Δx.Δv ≥h/4πmΔx≥h/4πmΔv≥6.6×10-34/4×3.14×10×5.25×10–26≥ 0.10×10–2m≥ 1×10–3 cmQ12) List the conditions wave function should satisfy?A12) The wave function, at a particular time, contains all the information that anybody at that time can have about the particle. But the wave function itself has no physical interpretation. It is not measurable. However, the square of the absolute value of the wave function has a physical interpretation. We interpret |ψ(x,t)|2 as a probability density, a probability per unit length of finding the particle at a time t at position x. The wave function ψ associated with a moving particle is not an observable quantity and does not have any direct physical meaning. It is a complex quantity. The complex wave function can be represented asψ(x, y, z, t) = a + iband its complex conjugate as ψ*(x, y, z, t) = a – ib. The product of wave function and its complex conjugate is ψ(x, y, z, t)ψ*(x, y, z, t) = (a + ib) (a – ib) = a2 + b2a2 + b2 is a real quantity. However, this can represent the probability density of locating the particle at a place in a given instant of time. The positive square root of ψ(x, y, z, t) ψ*(x, y, z, t) is represented as |ψ(x, y, z, t)|, called the modulus of ψ. The quantity |ψ(x, y, z, t)|2 is called the probability. This interpretation is possible because the product of a complex number with its complex conjugate is a real, non-negative number. We should be able to find the particle somewhere, we should only find it at one place at a particular instant, and the total probability of finding it anywhere should be one. For the probability interpretation to make sense, the wave function must satisfy certain conditions.
+ V(x) ψ ……….. (10) This is called the time-independent Schrodinger equation.The Schrodinger equation also known as Schrodinger’s wave equation is a partial differential equation that describes the dynamics of quantum mechanical systems by the wave function. The trajectory, the positioning, and the energy of these systems can be retrieved by solving the Schrodinger equation.All of the information for a subatomic particle is encoded within a wave function. The wave function will satisfy and can be solved by using the Schrodinger equation. The Schrodinger equation is one of the fundamental axioms that are introduced in undergraduate physics. Q9) The mass of an electron is 9.1×10–31 kg. Its uncertainty in velocity is 5.7×105 m/sec. Calculate uncertainty in its position?A9) Given m= 9×10–31ΔV= 5.7×105 m/sec Δx=?h= 6.6×10–34 Joule-Sec.Δx.Δv ≥h/4πmΔx≥h/4πmΔv≥6.6×10–34/9×3.14×9.1×10–31×5.7×105≥ 0.010×10–8≥ 1×10–10m Q10) The mass of a ball is 0.15 kg & its uncertainty in position to 10–10m. What is the value of uncertainty in its velocity?A10) Given m=0.15 kg. h=6.6×10-34 Joule-Sec.Δx = 10 –10 mΔv=?Δx.Δv ≥h/4πmΔv≥h/4πmΔx≥6.6×10-34/4×3.14×0.15×10–10≥ 3.50×10–24m Q11) The mass of a bullet is 10gm & uncertainty in its velocity is 5.25×10–26 cm/sec. Calculate the uncertainty in its position?A11) m=10 gm. h=6.6×10–27 erg-secΔv= 5.25×10–26 cmΔx=?Δx.Δv ≥h/4πmΔx≥h/4πmΔv≥6.6×10-34/4×3.14×10×5.25×10–26≥ 0.10×10–2m≥ 1×10–3 cmQ12) List the conditions wave function should satisfy?A12) The wave function, at a particular time, contains all the information that anybody at that time can have about the particle. But the wave function itself has no physical interpretation. It is not measurable. However, the square of the absolute value of the wave function has a physical interpretation. We interpret |ψ(x,t)|2 as a probability density, a probability per unit length of finding the particle at a time t at position x. The wave function ψ associated with a moving particle is not an observable quantity and does not have any direct physical meaning. It is a complex quantity. The complex wave function can be represented asψ(x, y, z, t) = a + iband its complex conjugate as ψ*(x, y, z, t) = a – ib. The product of wave function and its complex conjugate is ψ(x, y, z, t)ψ*(x, y, z, t) = (a + ib) (a – ib) = a2 + b2a2 + b2 is a real quantity. However, this can represent the probability density of locating the particle at a place in a given instant of time. The positive square root of ψ(x, y, z, t) ψ*(x, y, z, t) is represented as |ψ(x, y, z, t)|, called the modulus of ψ. The quantity |ψ(x, y, z, t)|2 is called the probability. This interpretation is possible because the product of a complex number with its complex conjugate is a real, non-negative number. We should be able to find the particle somewhere, we should only find it at one place at a particular instant, and the total probability of finding it anywhere should be one. For the probability interpretation to make sense, the wave function must satisfy certain conditions. 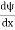 must also be continuous everywhere except where V(x) is infinite.
must also be continuous everywhere except where V(x) is infinite. 0 as x
0 as x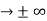 .
.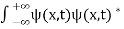 dx =1 Only wave function with all these properties can yield physically meaningful result. Q13) What is Physical significance of wave function?A13)
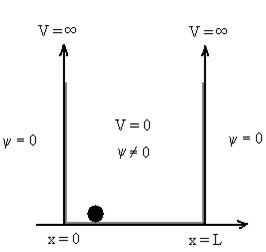
dx =1 Only wave function with all these properties can yield physically meaningful result. Q13) What is Physical significance of wave function?A13)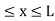 ………. (1)V=
………. (1)V= 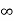 for x <0, x>L ………. (2)
for x <0, x>L ………. (2)
|
 ψ +
ψ +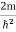 Eψ =0 …………….(2) Substituting
Eψ =0 …………….(2) Substituting 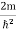 E = k2 …………….(3) writing the SWE for 1-D we get
E = k2 …………….(3) writing the SWE for 1-D we get 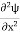 + k2 ψ =0 …………….(4) The general equation of above equation may be expressed asψ = Asin (kx + ϕ) …………….(5)Where A and ϕ are constants to be determined by boundary conditions Condition I: We have ψ = 0 at x = 0, therefore from equation0 = A sinϕ As A
+ k2 ψ =0 …………….(4) The general equation of above equation may be expressed asψ = Asin (kx + ϕ) …………….(5)Where A and ϕ are constants to be determined by boundary conditions Condition I: We have ψ = 0 at x = 0, therefore from equation0 = A sinϕ As A 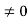 then sinϕ =0 or ϕ=0 …………….(6)Condition II:Further ψ = 0 at x = L, and ϕ=0, therefore from equation (5)0 = Asin kL As A
then sinϕ =0 or ϕ=0 …………….(6)Condition II:Further ψ = 0 at x = L, and ϕ=0, therefore from equation (5)0 = Asin kL As A 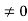 then sinkL =0 or kL=nπk =
then sinkL =0 or kL=nπk = 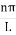 …………….(7)where n= 1,2,3,4………Substituting the value of k from (7) to (3)
…………….(7)where n= 1,2,3,4………Substituting the value of k from (7) to (3) 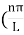 )2 =
)2 = 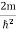 E This gives energy of level En =
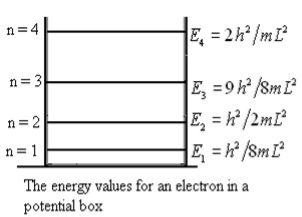
E This gives energy of level En =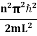 n=1,2,3,4…so on …………….(8)
n=1,2,3,4…so on …………….(8) From equation En is the energy value (Eigen Value) of the particle in a well. It is clear that the energy values of the particle in well are discrete not continuous.
|
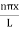 …………….(9)The probability density |ψ(x,t)|2 = ψ ψ*|ψ(x,t)|2= A2sin2
…………….(9)The probability density |ψ(x,t)|2 = ψ ψ*|ψ(x,t)|2= A2sin2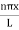 …………….(10)The probability density is zero at x = 0 and x = L. since the particle is always within the well
…………….(10)The probability density is zero at x = 0 and x = L. since the particle is always within the well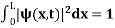 …………….(11)
…………….(11)
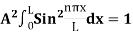
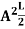 =1A =
=1A = 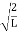 Substituting A in equation (9) we getψ =ψn=
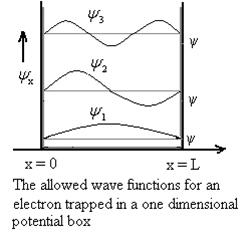
Substituting A in equation (9) we getψ =ψn= 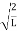 sin
sin n=1,2,3,4….. …………….(12)The above equation (12) is normalized wave function or Eigen function belonging to energy value En
n=1,2,3,4….. …………….(12)The above equation (12) is normalized wave function or Eigen function belonging to energy value En
|
|
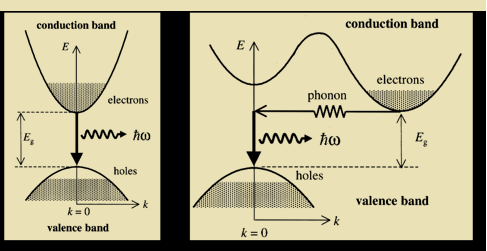
 1031, the quantum nature of this radiation is unimportant. As a result, this radiation can be treated fairly accurately by the classical theory of electromagnetism.Q16) Consider a one-dimensional particle which is confined within the region 0
1031, the quantum nature of this radiation is unimportant. As a result, this radiation can be treated fairly accurately by the classical theory of electromagnetism.Q16) Consider a one-dimensional particle which is confined within the region 0  x
x a and whose wave function is (x, t) =sin(πx/a) exp-iωt.(a) Find the potential V(x).(b) Calculate the probability of finding the particle in the interval a/4
a and whose wave function is (x, t) =sin(πx/a) exp-iωt.(a) Find the potential V(x).(b) Calculate the probability of finding the particle in the interval a/4  x
x  3a/4.A16) Since the first time derivative and the second x derivative of (x, t), are given by
3a/4.A16) Since the first time derivative and the second x derivative of (x, t), are given by 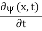 =-iω (x, t) and
=-iω (x, t) and 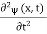 =-(π2/a2) (x, t)the Schrödinger equation yields
=-(π2/a2) (x, t)the Schrödinger equation yields
|
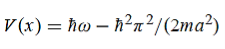 (b) The probability of finding the particle in the interval a/4
(b) The probability of finding the particle in the interval a/4 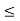 x
x 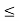 3a/4.can be obtained from
3a/4.can be obtained from
|
|
|
 where A and k are constants. Find the explicit form of the potential V (x).A19)
where A and k are constants. Find the explicit form of the potential V (x).A19)
|
|
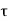 is the probability that the particle lies in the volume element d
is the probability that the particle lies in the volume element d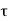 located at r at time t
located at r at time t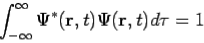 It is customary to also normalize many-particle wavefunctions to 1. The wavefunction must also be single-valued, continuous, and finite. 2. To every observable in classical mechanics, there corresponds a linear, Hermitian operator in quantum mechanics. For example, in coordinate space, the momentum operator
It is customary to also normalize many-particle wavefunctions to 1. The wavefunction must also be single-valued, continuous, and finite. 2. To every observable in classical mechanics, there corresponds a linear, Hermitian operator in quantum mechanics. For example, in coordinate space, the momentum operator 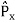 corresponding to momentum px in the
corresponding to momentum px in the 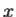 direction for a single particle is -iℏ
direction for a single particle is -iℏ . 3. The average value of the observable corresponding to operator
. 3. The average value of the observable corresponding to operator 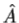 is given by
is given by 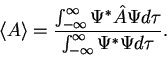 4. The wavefunction evolves in time according to the time-dependent Schrödinger equation
4. The wavefunction evolves in time according to the time-dependent Schrödinger equation 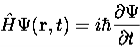 5. In any measurement of the observable associated with operator
5. In any measurement of the observable associated with operator 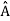 , the only values that will ever be observed are the eigenvalues a which satisfy
, the only values that will ever be observed are the eigenvalues a which satisfy 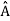 = a. Although measurements must always yield an eigenvalue, the state does not originally have to be in an eigenstate of
= a. Although measurements must always yield an eigenvalue, the state does not originally have to be in an eigenstate of 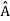 . An arbitrary state can be expanded in the complete set of eigenvectors of
. An arbitrary state can be expanded in the complete set of eigenvectors of 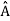 (
( = a) as, =
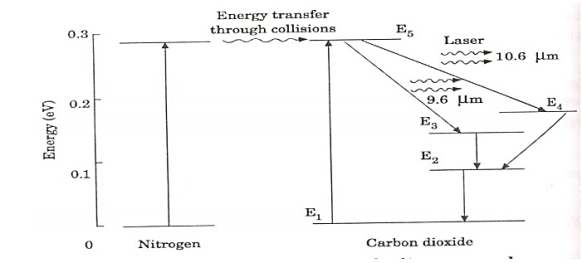
= a) as, = 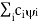 where the sum can run to infinity in principle. The probability of observing eigenvalue ai is given by ci*ci. 6. The total wavefunction must be antisymmetric with respect to the interchange of all coordinates of one fermion with those of another. Electronic spin must be included in this set of coordinates. The Pauli Exclusion Principle is a direct result of this antisymmetry principle. Q21) Discuss principle, construction and working of CO2 Laser?A21) CO2 LASERIn a molecular gas laser, laser action is achieved by transitions between vibrational and rotational levels of molecules. Its construction is simple and the output of this laser is continuous. In CO2 molecular gas laser, transition takes place between the vibrational states of Carbon dioxide molecules. CO2 Molecular gas laser It was the first molecular gas laser developed by Indian born American scientist Prof.C.K.N.Pillai. It is a four level laser and it operates at 10.6 μm in the far IR region. It is a very efficient laser. Energy states of CO2 molecules.A carbon dioxide molecule has a carbon atom at the center with two oxygen atoms attached, one at both sides. Such a molecule exhibits three independent modes of vibrations. They are a) Symmetric stretching mode. b) Bending mode c) Asymmetric stretching mode.a. Symmetric stretching modeIn this mode of vibration, carbon atoms are at rest and both oxygen atoms vibrate simultaneously along the axis of the molecule departing or approaching the fixed carbon atoms.
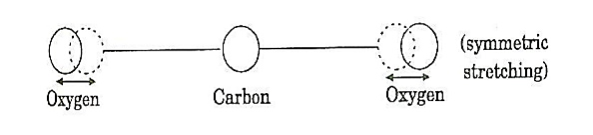
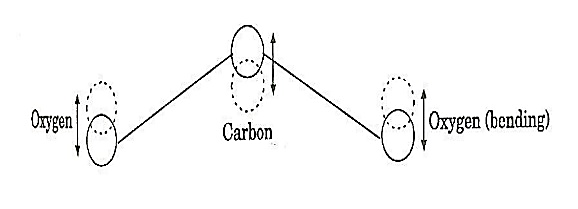
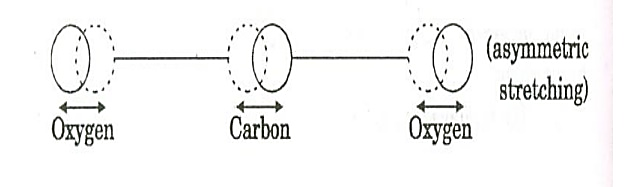
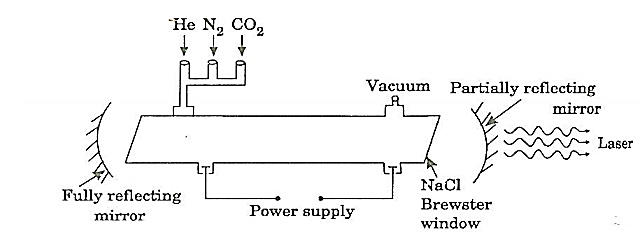
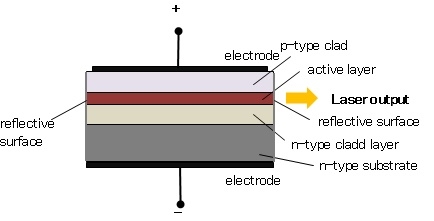
where the sum can run to infinity in principle. The probability of observing eigenvalue ai is given by ci*ci. 6. The total wavefunction must be antisymmetric with respect to the interchange of all coordinates of one fermion with those of another. Electronic spin must be included in this set of coordinates. The Pauli Exclusion Principle is a direct result of this antisymmetry principle. Q21) Discuss principle, construction and working of CO2 Laser?A21) CO2 LASERIn a molecular gas laser, laser action is achieved by transitions between vibrational and rotational levels of molecules. Its construction is simple and the output of this laser is continuous. In CO2 molecular gas laser, transition takes place between the vibrational states of Carbon dioxide molecules. CO2 Molecular gas laser It was the first molecular gas laser developed by Indian born American scientist Prof.C.K.N.Pillai. It is a four level laser and it operates at 10.6 μm in the far IR region. It is a very efficient laser. Energy states of CO2 molecules.A carbon dioxide molecule has a carbon atom at the center with two oxygen atoms attached, one at both sides. Such a molecule exhibits three independent modes of vibrations. They are a) Symmetric stretching mode. b) Bending mode c) Asymmetric stretching mode.a. Symmetric stretching modeIn this mode of vibration, carbon atoms are at rest and both oxygen atoms vibrate simultaneously along the axis of the molecule departing or approaching the fixed carbon atoms.
|
|
|
|
|
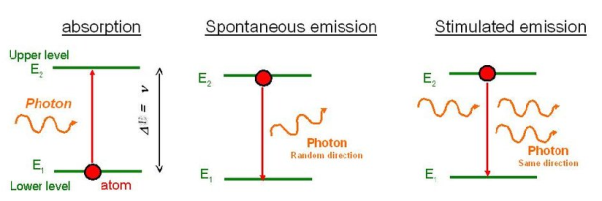
Spontaneous emission | Stimulated emission |
1.The spontaneous emission was postulated by Bohr | 1.The stimulated emission was postulated by Einstein |
2. Additional photons are not required in spontaneous emission | 2. Additional photons are required in stimulated emission |
3.One photon is emitted in spontaneous emission | 3.Two photons are emitted in stimulated emission |
4.The emitted radiation is poly-monochromatic | 4.The emitted radiation is monochromatic |
5. The emitted radiation is Incoherent | 5. The emitted radiation is Coherent |
6. The emitted radiation is less intense | 6. The emitted radiation is high intense |
7.The emitted radiation have less directionality | 7.The emitted radiation have high directionality |
8. Example: light from sodium or mercury lamp | 8. Example: light from laser source.
|
|
|
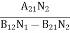 ………(5)Divide with B21N2 in numerator and denominator in right side of the above equation,ρ(υ) =
………(5)Divide with B21N2 in numerator and denominator in right side of the above equation,ρ(υ) = 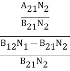 =
= 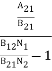 ………(6)ρ(υ) =
………(6)ρ(υ) = 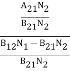 =
= 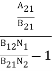 =
=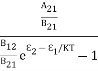 ………(7)We know from Maxwell Boltzmann distribution law
………(7)We know from Maxwell Boltzmann distribution law =
= 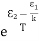 ………(8) And also from Planck’s law, the radiation densityρ(υ) =
………(8) And also from Planck’s law, the radiation densityρ(υ) = 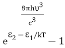 ………(9)Comparing the two equations (7) and (9)
………(9)Comparing the two equations (7) and (9)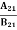 =
=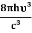 and
and  =1 ………(10)The above relations referred to as Einstein relations.From the above equation for non degenerate energy levels the stimulated emission rate is equal to the stimulated absorption rate at the equilibrium condition.
=1 ………(10)The above relations referred to as Einstein relations.From the above equation for non degenerate energy levels the stimulated emission rate is equal to the stimulated absorption rate at the equilibrium condition.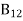 =
=  ………(11) Q26) What is the relationship between B21 and B12?
………(11) Q26) What is the relationship between B21 and B12?a) B12> B21
b) B12< B21
c) B12 = B21
d) No specific relation
A26) C is the correct answer.
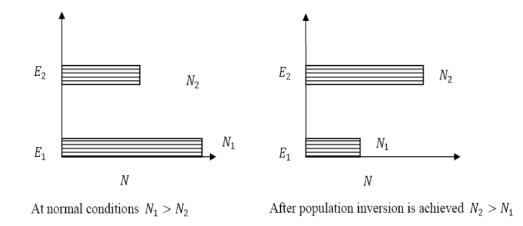
B21 is the coefficient for the stimulated emission while B12 is the coefficient for stimulated absorption. Both the processes are mutually reverse processes and their probabilities are equal. Therefore, B12 = B21. Q27) How laser light get amplify by population inversion ? A27) Population inversion DefinitionThe number of atoms present in the excited state or higher energy state is greater than the number of atoms present in the ground state or lower energy state is called population inversion. Population inversion as name suggests that this is inverted phenomena. In general lower energy level is more populated that means it have more number of atoms in lower energy level as compared to higher energy level. But by pumping we will obtain a state when the number of atoms present in the higher energy state is greater than the number of atoms present in lower energy state. Let us consider two level energy system of energies E1 and E2 as shown in figure. Let N1 and N2 be the populations that means number of atoms per unit volume of energy levels E1 andE2 respectively. According to Boltzmann’s distribution the population of an energy level E, at temperature T is given by Ni=N0
 Where N0 is the population of the lower level or ground state and k is the Boltzmann’s constant. From the above relation, the population of energy levels E1 andE2 are N1=N0
Where N0 is the population of the lower level or ground state and k is the Boltzmann’s constant. From the above relation, the population of energy levels E1 andE2 are N1=N0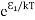 N2=N0
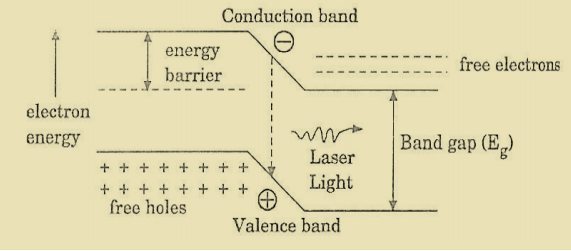
N2=N0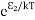 At ordinary conditionsN1>N2 i.e., the population in the ground or lower state is always greater than the population in the excited or higher states. The stage of making, population of higher energy level is greater than the population of lower energy level is called population inversion i.e. N1< N2
At ordinary conditionsN1>N2 i.e., the population in the ground or lower state is always greater than the population in the excited or higher states. The stage of making, population of higher energy level is greater than the population of lower energy level is called population inversion i.e. N1< N2
|


|
|
|
|